question_answer 1) A circular coil has 500 turn of wire and its radius is 5 cm. The self-inductance of the coil is
A)
\[25\times {{10}^{-}}^{3}mH\]
done
clear
B)
\[25mH\]
done
clear
C)
\[50\times {{10}^{-}}^{3}H\]
done
clear
D)
\[50\times {{10}^{-}}^{3}mH\]
done
clear
View Answer play_arrow
question_answer 2) An astronomical telescope has a large aperture to
A)
reduce spherical aberration
done
clear
B)
have high resolution
done
clear
C)
increase span of observation
done
clear
D)
have low dispersion
done
clear
View Answer play_arrow
question_answer 3) Which of the following is more close to a black body?
A)
Black board paint
done
clear
B)
Green leaves
done
clear
C)
Black holes
done
clear
D)
Red roses
done
clear
View Answer play_arrow
question_answer 4) A child swinging on a swing in sitting position, stands up, then the time period of swing will
A)
increase
done
clear
B)
decrease
done
clear
C)
remain same
done
clear
D)
increase if the child is long and decrease if the child is short
done
clear
View Answer play_arrow
question_answer 5) The radius of nucleus of silver (atomic number =47) is \[3.4\times {{10}^{-14}}m.\]The electric potential on the surface of nucleus is \[(e=1.6\times {{10}^{-19}}C)\]
A)
\[1.99\times {{10}^{6}}V\]
done
clear
B)
\[2.9\times {{10}^{6}}V\]
done
clear
C)
\[4.99\times {{10}^{6}}V\]
done
clear
D)
\[0.99\times {{10}^{6}}V\]
done
clear
View Answer play_arrow
question_answer 6) The unit of inductance is
A)
\[\frac{\text{volt}}{\text{ampere}}\]
done
clear
B)
\[\frac{\text{joule}}{\text{ampere}}\]
done
clear
C)
\[\frac{\text{volt-second}}{\text{ampere}}\]
done
clear
D)
\[\frac{\text{volt-ampere}}{\text{second}}\]
done
clear
View Answer play_arrow
question_answer 7) If a body travels half the distance with velocity \[{{v}_{1}}\] and the next half with velocity \[{{v}_{2}}\]its average velocity will be given by
A)
\[v=\sqrt{{{v}_{1}}{{v}_{2}}}\]
done
clear
B)
\[v=\frac{{{v}_{1}}+{{v}_{1}}}{2}\]
done
clear
C)
\[v=\frac{{{v}_{1}}}{{{v}_{2}}}\]
done
clear
D)
\[\frac{2}{v}=\frac{1}{{{v}_{1}}}+\frac{1}{{{v}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 8) The ratio of the numerical value of the average velocity and average speed of body is always
A)
unity
done
clear
B)
unity or less
done
clear
C)
unity or more
done
clear
D)
less than unity
done
clear
View Answer play_arrow
question_answer 9) Mark the correct option. If a ball is projected with velocity \[{{v}_{o}}\] at an angle of elevation 30°, then
A)
gravitational potential energy will be minimum at the highest point of the trajectory
done
clear
B)
kinetic energy will be zero at the highest point of the trajectory
done
clear
C)
vertical component of momentum will be conserved
done
clear
D)
horizontal component of momentum will be conserved
done
clear
View Answer play_arrow
question_answer 10) A spring scale is adjusted to read zero. Particles of mass 1 g fall on the pan of the scale and collide elastically and they rebound upward with the same speed. If the height of fall of panicles is 2 m and their rate of collision is 100 particles per second, then the scale reading in grams will be \[(g=9.8m/{{s}^{2}})\]
A)
1000 g
done
clear
B)
1100g
done
clear
C)
1200 g
done
clear
D)
1252 g
done
clear
View Answer play_arrow
question_answer 11) A smooth sphere of mass m moving with velocity u directly collides elastically with another sphere of mass M at rest. After collision their final velocities are V and v respectively. The value of v is
A)
\[2u\frac{M}{m}\]
done
clear
B)
\[2u\frac{m}{M}\]
done
clear
C)
\[\frac{2u}{1+\frac{m}{M}}\]
done
clear
D)
\[\frac{2u}{1+\frac{M}{m}}\]
done
clear
View Answer play_arrow
question_answer 12)
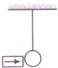
A block of mass 2 kg is kept on the floor. The coefficient of static friction is 0.4. If a force F of 2.5 N is applied on the block as shown in the figure, the frictional force between the block and the floor will be
A)
2.5 N
done
clear
B)
b) 5N
done
clear
C)
7.84 N
done
clear
D)
10N
done
clear
View Answer play_arrow
question_answer 13)
A mass of 10 g moving horizontally with a velocity of 100 cm/s strikes a pendulum bob of mass 10 g. The two masses stick together. The maximum height reached by the system now is (.g = 10 m/s2)
A)
zero
done
clear
B)
5 cm
done
clear
C)
2.5cm
done
clear
D)
1.25cm
done
clear
View Answer play_arrow
question_answer 14) The moments of inertia of two freely rotating bodies A and B are \[{{I}_{A}}\] and \[{{I}_{B}}\] respectively \[{{I}_{A}}>{{I}_{B}}\]and their angular momenta are equal. If \[{{K}_{A}}\] and \[{{K}_{B}}\] are their kinetic energies then
A)
\[{{K}_{A}}={{K}_{B}}\]
done
clear
B)
\[{{K}_{A}}>{{K}_{B}}\]
done
clear
C)
\[{{K}_{A}}<{{K}_{B}}\]
done
clear
D)
\[{{K}_{A}}=2{{K}_{B}}\]
done
clear
View Answer play_arrow
question_answer 15) A wheel of car is revolving at the rate of 1200 cycle/min. On pressing the accelerator after 10s, it does 4500 cycle/min then radial acceleration of the wheel is
A)
30rad/s2
done
clear
B)
1880 degree/s2
done
clear
C)
40 rad/s2
done
clear
D)
1980 degree/s2
done
clear
View Answer play_arrow
question_answer 16) The diameter of each plate of an air capacitor is 4 cm. To make the capacity of this plate capacitor equal to that of 20 cm diameter sphere, the distance between the plates will be
A)
\[4\times {{10}^{-3}}m\]
done
clear
B)
\[1\times {{10}^{-3}}m\]
done
clear
C)
1cm
done
clear
D)
\[1\times {{10}^{-3}}cm\]
done
clear
View Answer play_arrow
question_answer 17) The electric potential V is given as a function of distance \[x\] (m) by \[V=({{5.}^{2}}+10x-9)\]volt. Value of electric field at \[x\] = 1 m is
A)
20V/m
done
clear
B)
6V/m
done
clear
C)
11 V/m
done
clear
D)
-23V/m
done
clear
View Answer play_arrow
question_answer 18) In Young's experiment, the wavelength of red light is 7800Å and that of blue light is 5200Å. The value of n for which (n +1) the blue band coincides with nth red band is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 19) If the height of a satellite from the earth is negligible in comparison to the radius of the earth R, the orbital velocity of the satellite is
A)
\[gR\]
done
clear
B)
\[\frac{gR}{2}\]
done
clear
C)
\[\sqrt{\frac{g}{R}}\]
done
clear
D)
\[\sqrt{gR}\]
done
clear
View Answer play_arrow
question_answer 20) A satellite is revolving round me earth in an elliptical orbit. Its speed
A)
will be same at all points of the orbit
done
clear
B)
will be maximum when it is at maximum distance from earth
done
clear
C)
will be maximum when its distance from the earth will be minimum
done
clear
D)
goes on increasing or decreasing continuous in dependines upon the mass of the satellite
done
clear
View Answer play_arrow
question_answer 21) A particle of mass 10 g is executing simple harmonic motion with an amplitude of 0.5 m and periodic time of (\[\pi /5\]) second. The maximum value of the force acting on the particle is
A)
25 N
done
clear
B)
5N
done
clear
C)
2.5 N
done
clear
D)
0.5 N
done
clear
View Answer play_arrow
question_answer 22) The period of vibration of a mass m suspended from a spring is 2 s. If along with it another mass of 2 kg is also suspended, the period of oscillation increases by Is. The mass m will be
A)
2 kg
done
clear
B)
1 kg
done
clear
C)
1.6kg
done
clear
D)
2.6kg
done
clear
View Answer play_arrow
question_answer 23) A wire of length L and cross-sectional area A is made of a material of Young's modulus Y. It is stretched by an amount x. The work done is
A)
\[\frac{YxA}{2L}\]
done
clear
B)
\[\frac{Y{{x}^{2}}A}{L}\]
done
clear
C)
\[\frac{Y{{x}^{2}}A}{2L}\]
done
clear
D)
\[\frac{2Y{{x}^{2}}A}{L}\]
done
clear
View Answer play_arrow
question_answer 24) Compressibility of water is \[5\times {{10}^{-10}}m/N.\] The change in volume of 100 mL water subjected to \[15\times {{10}^{6}}\] Pa pressure will
A)
no change
done
clear
B)
increase by 0.98 mL
done
clear
C)
decrease by 0.75 mL
done
clear
D)
increase by 1.50 mL
done
clear
View Answer play_arrow
question_answer 25) A water film is made between two 10 cm long straight wires and at a distance of 0.5 cm. If distance between the wires is increased by 1 mm, then work done will be\[(T=7.2\times {{10}^{-2}}\,N/m)\]
A)
\[7.22\times {{10}^{-5}}J\]
done
clear
B)
\[1.44\times {{10}^{-5}}J\]
done
clear
C)
\[2.88\times {{10}^{-5}}J\]
done
clear
D)
\[5.76\times {{10}^{-5}}J\]
done
clear
View Answer play_arrow
question_answer 26) The force of cohesion is
A)
maximum in solids
done
clear
B)
maximum in liquids
done
clear
C)
maximum in gases
done
clear
D)
same in solid, liquid and gas
done
clear
View Answer play_arrow
question_answer 27) The temperature at which root mean square velocity of molecules of helium is equal to root mean square velocity of hydrogen at NTP is
A)
273°C
done
clear
B)
273 K
done
clear
C)
546°C
done
clear
D)
844 K
done
clear
View Answer play_arrow
question_answer 28) The average kinetic energy of a hydrogen gas molecule at NTP will be (Boltzmann's constant \[k=1.38\times {{10}^{-23}}J/K\]
A)
\[0.186\times {{10}^{-20}}J\]
done
clear
B)
\[0.372\times {{10}^{-20}}J\]
done
clear
C)
\[0.56\times {{10}^{-20}}J\]
done
clear
D)
\[5.6\times {{10}^{-20}}J\]
done
clear
View Answer play_arrow
question_answer 29) The height of a water fall is 84 m. Assuming that the entire kinetic energy of falling water is converted into heat, the rise in temperature of the water will be
A)
0.196°C
done
clear
B)
1.960°C
done
clear
C)
0.96°C
done
clear
D)
0.0196°C
done
clear
View Answer play_arrow
question_answer 30) For adiabatic process \[\left( \gamma \frac{{{C}_{p}}}{{{C}_{v}}} \right)\]
A)
\[{{p}^{\gamma }}V=\] constant
done
clear
B)
\[T{{p}^{\gamma }}V=\] constant
done
clear
C)
\[p{{V}^{\gamma }}=\]constant
done
clear
D)
\[pV=\] constant
done
clear
View Answer play_arrow
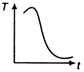
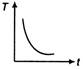
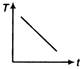
question_answer 31) A cup of tea having the temperature 80°C is kept in a room, which is at 20°C. Which of the given curves best represents the variation of temperature T of the cup with time r?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 32) An ice box made of Styrofoam (thermal conductivity \[=0.01J{{m}^{=1}}K)\] is used to keep liquids cool. It has a total wall area including lid of0.8 m2 and wall thickness of 2.0 cm. A bottle of water is placed in the box and filled with ice. If the outside temperature is 30°C, the rate of flow of, heat into the box is (in Js-1)
A)
16
done
clear
B)
14
done
clear
C)
12
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 33) The length of two open organ pipes are \[l\] and \[(l+\Delta l)\] respectively. Neglecting end corrections the beats frequency between them will be approximately
A)
\[\frac{v}{2l}\]
done
clear
B)
\[\frac{v}{4l}\]
done
clear
C)
\[\frac{v\Delta l}{2{{l}^{2}}}\]
done
clear
D)
\[\frac{v\Delta l}{l}\]
done
clear
View Answer play_arrow
question_answer 34) 5 g of ice of \[0{}^\circ C\] is dropped in a beaker containing 20 g of water at \[40{}^\circ C\]. The final temperature will be
A)
\[32{}^\circ C\]
done
clear
B)
\[16{}^\circ C\]
done
clear
C)
\[8{}^\circ C\]
done
clear
D)
\[24{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 35) If a body loses half of its velocity on penetrating 3 cm in a wooden block then how much will it penetrate more before coming to rest?
A)
1 cm
done
clear
B)
2 cm
done
clear
C)
3 cm
done
clear
D)
4 cm
done
clear
View Answer play_arrow
question_answer 36) The minimum velocity (in ms-1) with which a car driver must traverse a flat curve of radius 150 m and coefficient of friction to avoid skidding is
A)
60
done
clear
B)
30
done
clear
C)
15
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 37) The energy band gap is maximum in
A)
metals
done
clear
B)
superconductors
done
clear
C)
insulators
done
clear
D)
semiconductors
done
clear
View Answer play_arrow
question_answer 38) The phenomenon of polarization of light indicates that
A)
light is a longitudinal waves
done
clear
B)
light is transverse wave
done
clear
C)
light if not a wave
done
clear
D)
light travels with the velocity of\[~3\times {{10}^{8}}m/s\]
done
clear
View Answer play_arrow
question_answer 39) The manifestation of band structure in solids is due to
A)
Heisenberg's uncertainly principle
done
clear
B)
Pauli's exclusion principle
done
clear
C)
Bohr's correspondence principle
done
clear
D)
Boltzmann's law
done
clear
View Answer play_arrow
question_answer 40) If there were no gravity, which of the following will not be there for a fluid?
A)
Viscosity
done
clear
B)
Surface tension
done
clear
C)
Pressure
done
clear
D)
Archimedes' upward thrust
done
clear
View Answer play_arrow
question_answer 41) Which one of the following is ferromagnetic?
A)
Gold
done
clear
B)
Wood
done
clear
C)
Manganese
done
clear
D)
Nickle
done
clear
View Answer play_arrow
question_answer 42) Two lenses have powers equal to +2 D and -4 D respectively, the power of combination will be
A)
-2D
done
clear
B)
+2D
done
clear
C)
-40
done
clear
D)
+4D
done
clear
View Answer play_arrow
question_answer 43) Shown below is a distribution of charges, the flux of electric field due to these charges through the surface is
A)
\[\frac{3q}{{{\varepsilon }_{o}}}\]
done
clear
B)
zero
done
clear
C)
\[\frac{2q}{{{\varepsilon }_{o}}}\]
done
clear
D)
\[\frac{q}{{{\varepsilon }_{o}}}\]
done
clear
View Answer play_arrow
question_answer 44) A steel ball is dropped from a height h and it makes a perfectly elastic collision with a horizontal plane. Following its initial release, it will make periodic motion with frequency
A)
\[2\pi \sqrt{\frac{g}{h}}\]
done
clear
B)
\[2\pi \sqrt{\frac{h}{g}}\]
done
clear
C)
\[\frac{1}{2}\sqrt{\frac{g}{2h}}\]
done
clear
D)
\[\frac{1}{2}\sqrt{\frac{2h}{g}}\]
done
clear
View Answer play_arrow
question_answer 45) Three charged particles are initially in position-1. They are free to move and they come to position-2, after some time. Let \[{{U}_{1}}\] and \[{{U}_{2}}\] be the electrostatic potential energies in position-1 and 2 then
A)
\[{{U}_{1}}={{U}_{2}}\]
done
clear
B)
\[{{U}_{2}}\ge {{U}_{1}}\]
done
clear
C)
\[{{U}_{2}}>{{U}_{1}}\]
done
clear
D)
\[{{U}_{1}}>{{U}_{2}}\]
done
clear
View Answer play_arrow
question_answer 46) In Young's double slit experiment are slit is covered with red filter and another slit is covered by green filter, then interference pattern will be
A)
red
done
clear
B)
green
done
clear
C)
yellow
done
clear
D)
invisible
done
clear
View Answer play_arrow
question_answer 47) For the stationary wave \[y=u\,\sin \,\frac{\pi x}{15}\cos \,96\,\pi t\] The distance between a node and the next antinode is
A)
7.5cm
done
clear
B)
15cm
done
clear
C)
22.5 cm
done
clear
D)
30 cm
done
clear
View Answer play_arrow
question_answer 48) In L-R circuit the current increases to three- fourth of its maximum value in 4s, then the time constant of the circuit is
A)
\[2\,{{\log }_{e}}\,2\]
done
clear
B)
\[\frac{4}{\log {{\,}_{e}}\,2\,}\]
done
clear
C)
\[\frac{2}{\log {{\,}_{e}}\,2\,}\]
done
clear
D)
\[\frac{\log {{\,}_{e}}\,2\,}{2}\]
done
clear
View Answer play_arrow
question_answer 49) 1 Wb/m2 is equal to
A)
104 G
done
clear
B)
102 G
done
clear
C)
10-2 G
done
clear
D)
10-4 G
done
clear
View Answer play_arrow
question_answer 50) If the earth is treated as a sphere of radius R and mass M, its angular momentum about the axis of rotation with time period T is
A)
\[\frac{\pi M{{R}^{2}}\,}{T}\]
done
clear
B)
\[\frac{M{{R}^{2}}T\,}{2\pi }\]
done
clear
C)
\[\frac{2\pi M{{R}^{2}}\,}{T}\]
done
clear
D)
\[\frac{4\pi M{{R}^{2}}\,}{5T}\]
done
clear
View Answer play_arrow
question_answer 51) Which one of the following is used as a moderator in nuclear reaction?
A)
Uranium
done
clear
B)
Heavy water
done
clear
C)
Cadmium
done
clear
D)
Plutonium
done
clear
View Answer play_arrow
question_answer 52) The sensitiveness of a moving coil galvanometer can be increased by decreasing the
A)
number of turns in the coils
done
clear
B)
are of the coil
done
clear
C)
magnetic field
done
clear
D)
couple per unit twist of the suspension
done
clear
View Answer play_arrow
question_answer 53) An electric heater of resistance 6H works for 10 min on a 60 V line. The energy liberated in this period of time is
A)
\[7.2\times {{10}^{3}}J\]
done
clear
B)
\[3.6\times {{10}^{s}}J\]
done
clear
C)
\[43.2\times {{10}^{4}}J\]
done
clear
D)
\[28.8\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 54) The material of wire of potentiometer is made of
A)
copper
done
clear
B)
steel
done
clear
C)
manganin
done
clear
D)
aluminium
done
clear
View Answer play_arrow
question_answer 55) The rest mass of the photon is
A)
zero
done
clear
B)
infinite
done
clear
C)
between zero and infinite
done
clear
D)
equal to that of electron
done
clear
View Answer play_arrow
question_answer 56) The \[{{k}_{\alpha }}\] line from molybdenum (atomic number =42) has a wavelength of 0.7078 \[\overset{o}{\mathop{\text{A}}}\,\]. The wavelength of \[{{k}_{\alpha }}\] line of zinc (atomic number = 30) will be
A)
1\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
1.3872\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
0.3541\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
0.5\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 57) Decay constant of radioactive element is \[1.5\times {{10}^{9}}\]Average age of element is
A)
\[1.5\times {{10}^{9}}\]
done
clear
B)
\[4.62\times {{10}^{8}}\]
done
clear
C)
\[6.67\times {{10}^{8}}\]
done
clear
D)
\[10.35\times {{10}^{8}}\]
done
clear
View Answer play_arrow
question_answer 58) A potential difference V is applied the ends of a copper wire of length\[l\]and diameter d. On doubling only d, drift velocity
A)
becomes two times
done
clear
B)
becomes half
done
clear
C)
does not change
done
clear
D)
becomes one-fourth
done
clear
View Answer play_arrow
question_answer 59) A steady current of 5 A is maintained for 45 min. During this time it deposits 4.572 g of zinc at the cathode of a voltmeter. ECE of zinc is
A)
\[3.387\times {{10}^{-4}}\,g/C\]
done
clear
B)
\[3.397\times {{10}^{-4}}\,g/C\]
done
clear
C)
\[3.384\times {{10}^{-3}}\,g/C\]
done
clear
D)
\[3.394\times {{10}^{-3}}\,g/C\]
done
clear
View Answer play_arrow
question_answer 60) The earth's magnetic induction at a certain point is \[7\times {{10}^{-5}}\] Wb/m2. This is to be annulled by the magnetic induction at the centre of a circular conducting loop of radius 5 cm. The required current in the loop is
A)
0.56 A
done
clear
B)
5.6 A
done
clear
C)
0.28 A
done
clear
D)
2.8 A
done
clear
View Answer play_arrow
question_answer 61) Which of the following electrolytic solutions has the least specific conductance?
A)
0.02 N
done
clear
B)
0.2 N
done
clear
C)
2 N
done
clear
D)
0.002 N
done
clear
View Answer play_arrow
question_answer 62) Which of the following can be measured by the Ostwald-Walker dynamic method?
A)
Relative lowering of vapour pressure
done
clear
B)
Lowering of vapour pressure
done
clear
C)
Vapour pressure of the solvent
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 63) One mole of which of the following has the highest entropy?
A)
Liquid nitrogen
done
clear
B)
Hydrogen gas
done
clear
C)
Mercury
done
clear
D)
Diamond
done
clear
View Answer play_arrow
question_answer 64) Catalytic dehydrogenation of a primary alcohol gives a
A)
secondary alcohol
done
clear
B)
aldehyde
done
clear
C)
ketone
done
clear
D)
ester
done
clear
View Answer play_arrow
question_answer 65) Methoxy methane and ethanol are
A)
position isomers
done
clear
B)
chain isomers
done
clear
C)
functional isomers
done
clear
D)
optical isomers
done
clear
View Answer play_arrow
question_answer 66) Amines behave as
A)
Lewis acid
done
clear
B)
Lewis base
done
clear
C)
aprotic acid
done
clear
D)
neutral compound
done
clear
View Answer play_arrow
question_answer 67) Dalda is prepared from oils by
A)
oxidation
done
clear
B)
reduction
done
clear
C)
hydrolysis
done
clear
D)
distillation
done
clear
View Answer play_arrow
question_answer 68) The chemical name of anisole is
A)
ethanoic acid
done
clear
B)
methoxy benzene
done
clear
C)
propanone
done
clear
D)
acetone
done
clear
View Answer play_arrow
question_answer 69) The number of disulphide linkages present in insulin are
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 70) Waxes are esters of
A)
glycerol
done
clear
B)
long chain alcohols
done
clear
C)
glycerol and fatty acid
done
clear
D)
long chain alcohols and long chain fatty acids
done
clear
View Answer play_arrow
question_answer 71) Among the following, which is least acidic?
A)
phenol
done
clear
B)
o-cresol
done
clear
C)
p-nitrophenol
done
clear
D)
p-chlorophenol
done
clear
View Answer play_arrow
question_answer 72) Which of the following organic compounds answers to both iodoform test and Fehling's test?
A)
Ethanol
done
clear
B)
Methanal
done
clear
C)
Ethanal
done
clear
D)
Propanone
done
clear
View Answer play_arrow
question_answer 73) Lanthanides and actinides are also called
A)
short periods
done
clear
B)
inner-transition elements
done
clear
C)
long periods
done
clear
D)
main transition elements
done
clear
View Answer play_arrow
question_answer 74) The hydrogen electrode is dipped in a solution of\[pH=3\]at\[25{}^\circ C\]. The potential of the cell would be (the value of 2.303 RT/F is 0.059 V)
A)
0.177V
done
clear
B)
0.087V
done
clear
C)
\[-0.177V\]
done
clear
D)
0.059V
done
clear
View Answer play_arrow
question_answer 75) 1 mole of\[{{H}_{2}}S{{O}_{4}}\]is mixed with 2 moles of\[NaOH\]. The heat evolved will be
A)
57.3 kJ
done
clear
B)
\[2\times 57.3\text{ }kJ\]
done
clear
C)
57.3/2 kJ
done
clear
D)
Cannot be predicted
done
clear
View Answer play_arrow
question_answer 76) Which of the following solutions will have the highest boiling point?
A)
\[0.1\text{ }M\text{ }FeC{{l}_{3}}\]
done
clear
B)
\[0.1M\,BaC{{l}_{2}}\]
done
clear
C)
\[0.1\text{ }M\text{ }NaCl\]
done
clear
D)
0.1 M urea
done
clear
View Answer play_arrow
question_answer 77) Azeotropic mixture of\[HCl\]and water has
A)
48%\[HCl\]
done
clear
B)
22.2%\[HCl\]
done
clear
C)
36%\[HCl\]
done
clear
D)
20.2%\[HCl\]
done
clear
View Answer play_arrow
question_answer 78) One mole of a perfect gas expands isothermally to ten times of its original volume. The change in entropy is
A)
0.1R
done
clear
B)
2.303 R
done
clear
C)
10.0 R
done
clear
D)
100.0 R
done
clear
View Answer play_arrow
question_answer 79) For the reaction,\[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}};\] \[\Delta H=?\]
A)
\[\Delta E+2RT\]
done
clear
B)
\[\Delta F-2RT\]
done
clear
C)
\[\Delta F+RT\]
done
clear
D)
\[\Delta E-RT\]
done
clear
View Answer play_arrow
question_answer 80) 100 cc of\[0.6\text{ }N\text{ }{{H}_{2}}S{{O}_{4}}\]and 200 cc of\[0.3\text{ }N\text{ }HCl\]were mixed together. The normality of the solution will be
A)
0.2 N
done
clear
B)
0.4 N
done
clear
C)
0.8 N
done
clear
D)
0.6 N
done
clear
View Answer play_arrow
question_answer 81) The activation energy for most of the reactions is approximately\[50\text{ }kJ\text{ }mo{{l}^{-1}}\]. The value of temperature coefficient for such reactions is
A)
>2
done
clear
B)
>3
done
clear
C)
<1
done
clear
D)
>4
done
clear
View Answer play_arrow
question_answer 82) If the mass defect of\[_{4}^{9}X\]is 0.090 u, then binding energy per nucleon is (\[1u=9315MeV\])
A)
9.315 MeV
done
clear
B)
931.5 MeV
done
clear
C)
83.0 MeV
done
clear
D)
8.38 MeV
done
clear
View Answer play_arrow
question_answer 83) 50 mL of\[0.1\text{ }M\text{ }HCl\]and 50 mL of\[0.2\text{ }M\text{ }NaOH\]are mixed. The pH of the resulting solution is
A)
1.30
done
clear
B)
4.2
done
clear
C)
12.70
done
clear
D)
11.70
done
clear
View Answer play_arrow
question_answer 84) \[_{27}C{{o}^{60}}\] is radioactive because
A)
its atomic number is high
done
clear
B)
it has high\[\frac{p}{n}\] ratio
done
clear
C)
it has high \[\frac{n}{p}\]ratio
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 85) \[KI\] and\[CuS{{O}_{4}}\]solution when mixed gives
A)
\[Cu{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{u}_{2}}{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}+C{{u}_{2}}{{I}_{2}}+{{I}_{2}}\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}+Cu{{I}_{2}}+{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 86) Which of the following will form a colorless complex?
A)
\[N{{i}^{2+}}\]
done
clear
B)
\[C{{u}^{+}}\]
done
clear
C)
\[T{{i}^{2+}}\]
done
clear
D)
\[C{{u}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 87)
In the reaction,
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 88)
The IUPAC name of the following compound is
A)
propionic anhydride
done
clear
B)
dipropanoic anhydride
done
clear
C)
ethoxy propanoic acid
done
clear
D)
propanoic anhydride
done
clear
View Answer play_arrow
question_answer 89) Hinsberg reagent is
A)
\[{{C}_{6}}{{H}_{5}}S{{O}_{3}}H\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}NO\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}{{N}_{2}}Cl\]
done
clear
View Answer play_arrow
question_answer 90) Acetaldehyde cannot show
A)
iodoform test
done
clear
B)
Lucas test
done
clear
C)
Benedict's test
done
clear
D)
Tollen's test
done
clear
View Answer play_arrow
question_answer 91) Which of the following will be most readily dehydrated under acidic conditions?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 92) Which of the following cannot reduce Fehling solution?
A)
\[HCOOH\]
done
clear
B)
\[{{H}_{3}}CCOOH\]
done
clear
C)
\[HCHO\]
done
clear
D)
\[{{H}_{3}}CCHO\]
done
clear
View Answer play_arrow
question_answer 93) Absolute alcohol is prepared by
A)
vacuum distillation
done
clear
B)
azeotropic distillation
done
clear
C)
steam distillation
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 94) Which of the following compounds is resistant to nucleophilic attack by hydroxyl ion?
A)
Methylacetate
done
clear
B)
Acetonitrile
done
clear
C)
Acetamide
done
clear
D)
Diethyl ether
done
clear
View Answer play_arrow
question_answer 95) Ethyl amine reacts with nitrous acid to form
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH,{{N}_{2}},{{H}_{2}}O\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}N_{2}^{+}C{{l}^{-}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}NHOH,N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 96) Rice is deficient in
A)
lysine
done
clear
B)
alanine
done
clear
C)
glycine
done
clear
D)
leucine
done
clear
View Answer play_arrow
question_answer 97) Mutarotation does not occur in
A)
sucrose
done
clear
B)
D-glucose
done
clear
C)
L-glucose
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 98) Aldehyde which is formed during photo synthesis of plants is
A)
methanal
done
clear
B)
acetaldehyde
done
clear
C)
propanal
done
clear
D)
phenylmethanal
done
clear
View Answer play_arrow
question_answer 99)
A)
aspartame
done
clear
B)
cyclate
done
clear
C)
saccharin
done
clear
D)
valium
done
clear
View Answer play_arrow
question_answer 100) Bithional is an example of
A)
antiseptic
done
clear
B)
disinfectant
done
clear
C)
antibiotic
done
clear
D)
tranquiliser
done
clear
View Answer play_arrow
question_answer 101) Conjugate acid of\[C{{H}_{3}}N{{H}_{2}}\]is
A)
\[C{{H}_{3}}OH\]
done
clear
B)
\[C{{H}_{3}}\overset{+}{\mathop{N}}\,{{H}_{3}}\]
done
clear
C)
\[C{{H}_{2}}NH_{2}^{+}\]
done
clear
D)
\[C{{H}_{3}}\overset{-}{\mathop{N}}\,H\]
done
clear
View Answer play_arrow
question_answer 102) In nuclear explosion, the energy is released in the form of
A)
kinetic energy
done
clear
B)
electrical energy
done
clear
C)
potential energy
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 103) Phosgene is the common name of the following compound
A)
Mixture of\[C{{O}_{2}}\]and \[C{{l}_{2}}\]
done
clear
B)
\[POC{{l}_{3}}\]
done
clear
C)
Carbonyl chloride
done
clear
D)
Carbon tetrachloride
done
clear
View Answer play_arrow
question_answer 104)
A)
\[N{{O}_{2}}\]
done
clear
B)
\[NaN{{O}_{2}}\]
done
clear
C)
\[AgN{{O}_{2}}\]
done
clear
D)
\[NaN{{O}_{2}}+dil.HCl\]
done
clear
View Answer play_arrow
question_answer 105) The end product in the sequence would be \[{{C}_{3}}{{H}_{7}}OH\xrightarrow[{{170}^{o}}]{{{H}_{2}}S{{O}_{4}}}A\xrightarrow{B{{r}_{2}}}B\xrightarrow[KOH]{Alc.}C\]
A)
\[C{{H}_{3}}C\equiv CH\]
done
clear
B)
\[C{{H}_{3}}\text{ }\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,=C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{2}}=C=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}\text{ }\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,\]
done
clear
View Answer play_arrow
question_answer 106) The use of\[CC{{l}_{4}}\]as fire extinguisher depends on the fact that
A)
it is an inorganic compound
done
clear
B)
it is an organic compound
done
clear
C)
its vapour is much heavier than air and non-combustible
done
clear
D)
it is a compound of chlorine
done
clear
View Answer play_arrow
question_answer 107) Ether on carbonylation gives
A)
alkanoic acid
done
clear
B)
alkanone
done
clear
C)
alkyl alkanoate
done
clear
D)
alkanal
done
clear
View Answer play_arrow
question_answer 108) \[C{{H}_{3}}CHO+C{{H}_{3}}CHO\xrightarrow{Dil.O{{H}^{-}}}\]\[C{{H}_{3}}CH(OH)C{{H}_{2}}CHO,\]the intermediate in the above reaction is
A)
carbanion
done
clear
B)
carbonium ion
done
clear
C)
carbene
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 109) Ethanol shows intermolecular H-bonding. Which of the following property is affected by H-bonding?
A)
Volatility
done
clear
B)
Boiling point
done
clear
C)
Solubility
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 110) How many\[\alpha -\]hydrogens are present in diethyl ether?
A)
10
done
clear
B)
6
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 111) Which one of the following types of drugs reduces fever?
A)
Tranquiliser
done
clear
B)
Antibiotic
done
clear
C)
Antipyretic
done
clear
D)
Analgesic
done
clear
View Answer play_arrow
question_answer 112) Which of the following is a polyamide?
A)
Bakelite
done
clear
B)
Terylene
done
clear
C)
Nylon-66
done
clear
D)
Teflon
done
clear
View Answer play_arrow
question_answer 113) Tertiary alkyl halides are practically inert towards substitution by\[{{S}_{N}}2\]mechanism because of
A)
steric hindrance
done
clear
B)
inductive effect
done
clear
C)
instability
done
clear
D)
insolubility
done
clear
View Answer play_arrow
question_answer 114) Which belongs to the actinide series?
A)
\[Ce\]
done
clear
B)
\[Cf\]
done
clear
C)
\[Ca\]
done
clear
D)
\[Cs\]
done
clear
View Answer play_arrow
question_answer 115) For a first order reaction at\[27{}^\circ C,\]the ratio of time required for 75% completion to 25% completion of reaction is
A)
\[\log \frac{4}{3}\]
done
clear
B)
\[\log \frac{3}{4}\]
done
clear
C)
\[\frac{\log \,4}{\log 4/3}\]
done
clear
D)
\[\frac{\log \,4/3}{\log 4}\]
done
clear
View Answer play_arrow
question_answer 116) If the rate of reaction,\[A\to B\]doubles on increasing the concentration of A by 4 times, the order of the reaction is
A)
2
done
clear
B)
1
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 117) The group of elements in which the differentiating electrons enters in the antepenultimate shell of atoms are called
A)
\[f-\]block elements
done
clear
B)
\[p-\]block elements
done
clear
C)
\[s-\]block elements
done
clear
D)
\[d-\]block elements
done
clear
View Answer play_arrow
question_answer 118) Brass, bronze and german silver have one common metal. This is
A)
\[Zn\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Al\]
done
clear
D)
\[Cu\]
done
clear
View Answer play_arrow
question_answer 119) Which one of the following aqueous solutions of salts has the lowest pH value?
A)
\[C{{H}_{3}}COONa\]
done
clear
B)
\[NaCl\]
done
clear
C)
\[N{{H}_{4}}OOCC{{H}_{3}}\]
done
clear
D)
\[N{{H}_{4}}Cl\]
done
clear
View Answer play_arrow
question_answer 120) Select the statement which is not true?
A)
Nylon fibres are elastic.
done
clear
B)
Nylon fibres are not crease resistant.
done
clear
C)
Nylon fibres are used in parachute.
done
clear
D)
Both and .
done
clear
View Answer play_arrow
question_answer 121) Entamoeba gingivalis is causative agent of
A)
pyorrhea
done
clear
B)
amoebiasis
done
clear
C)
bronchitis
done
clear
D)
no disease but aggrevates pyorrhea
done
clear
View Answer play_arrow
question_answer 122) Post erythrocytic cycle of Plasmodium is also called
A)
Ross cycle
done
clear
B)
Krebs' cycle
done
clear
C)
WBCs cycle
done
clear
D)
Golgi cycle
done
clear
View Answer play_arrow
question_answer 123) Digestion in Leucosolenia and other sponges is
A)
only extracellular
done
clear
B)
only intracellular
done
clear
C)
first extracellular, then intracellular
done
clear
D)
first intracellular, then extracellular
done
clear
View Answer play_arrow
question_answer 124) A cysticerous in pig muscles can remain alive for
A)
six months
done
clear
B)
one year
done
clear
C)
six years
done
clear
D)
one month
done
clear
View Answer play_arrow
question_answer 125) Elephantiasis is caused by
A)
Entamoeba coli
done
clear
B)
trematodes
done
clear
C)
cestodes
done
clear
D)
Wuchereria bancrofti
done
clear
View Answer play_arrow
question_answer 126) Tube within tube plan is exhibited by which of the following groups of animals?
A)
Arthropods
done
clear
B)
Cephalochordates
done
clear
C)
Annelids
done
clear
D)
Mollusca
done
clear
View Answer play_arrow
question_answer 127) Molluscan blood contains
A)
haemoglobin
done
clear
B)
haemozoin.
done
clear
C)
haemocyanin
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 128) Male mosquito is not sanguivore as
A)
it lacks cutting and biting mandibles
done
clear
B)
it does not require any kind of proteins ?
done
clear
C)
it lacks smelling power
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 129) Moulting in snakes is done by shedding
A)
comified cells
done
clear
B)
stratified germinativum
done
clear
C)
epidermis
done
clear
D)
dermis
done
clear
View Answer play_arrow
question_answer 130) Leveret is young one of
A)
eagle
done
clear
B)
hawk
done
clear
C)
deer
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 131) Caudal vertebrae of man are united to form a single
A)
synsacrum
done
clear
B)
pygostyle
done
clear
C)
coccyx
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 132) Fluid mosaic model exhibits amphipathy because of
A)
glycoproteins
done
clear
B)
phospholipids
done
clear
C)
lipids
done
clear
D)
glycolipids
done
clear
View Answer play_arrow
question_answer 133) The repeating unit in glycogen is
A)
glucose
done
clear
B)
fructose
done
clear
C)
galactose
done
clear
D)
cellulose
done
clear
View Answer play_arrow
question_answer 134) Non-reducing sugars have
A)
free CHO group and bound CO group
done
clear
B)
free CO group and bound CHO group
done
clear
C)
both CO and CHO free groups
done
clear
D)
neither free CO nor free CHO groups
done
clear
View Answer play_arrow
question_answer 135) During mitosis nuclear membrane disappears at
A)
early prophase
done
clear
B)
late prophase
done
clear
C)
metaphase
done
clear
D)
anaphase
done
clear
View Answer play_arrow
question_answer 136) Rarely observed phenotype in population is called
A)
wild type
done
clear
B)
mutant type
done
clear
C)
variant type
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 137) If a boy's father has haemophilia and his mother has one gene for haemophilia, what is the chance that the boy will inherit the disease?
A)
100%
done
clear
B)
50%
done
clear
C)
0%
done
clear
D)
75%
done
clear
View Answer play_arrow
question_answer 138) The controlling centre of cell is
A)
nucleus
done
clear
B)
nucleolus
done
clear
C)
mitochondria
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 139) Which one of the following codons codes for the some information as UGC?
A)
UGU
done
clear
B)
UGA
done
clear
C)
UAG
done
clear
D)
UGG
done
clear
View Answer play_arrow
question_answer 140) In testis of frog, there are no
A)
Sertoli cells
done
clear
B)
spermatocytes
done
clear
C)
spermadds
done
clear
D)
intestinal ceils
done
clear
View Answer play_arrow
question_answer 141) Iron is stored in bone marrow as
A)
haemosedrin
done
clear
B)
ferritin
done
clear
C)
haematin
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 142) Red muscle cells are rich in
A)
only myosin
done
clear
B)
haemoglobin and glucose
done
clear
C)
lactic acid and acetic acid
done
clear
D)
myoglobin and cytochrome
done
clear
View Answer play_arrow
question_answer 143) Pepsin acts in:
A)
basic medium
done
clear
B)
acidic medium
done
clear
C)
neutral medium
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 144) One difference in breathing mechanism of frog and rabbit is that
A)
expansion of frog's lungs in not much
done
clear
B)
abdominal movements more in frog
done
clear
C)
diaphragm and rib muscles play important role in rabbit
done
clear
D)
air reaching frog's lungs is to cool
done
clear
View Answer play_arrow
question_answer 145) The movements of neutrophils to the site of inflammation is due to
A)
release of hormone
done
clear
B)
Chemotaxis
done
clear
C)
activity of antigens
done
clear
D)
activations of antigens
done
clear
View Answer play_arrow
question_answer 146) Kidney stones are crystals of
A)
sodium chloride
done
clear
B)
silica
done
clear
C)
calcium oxalate
done
clear
D)
potassium chloride
done
clear
View Answer play_arrow
question_answer 147) All the following are the properties of skeletal muscles except
A)
excitability
done
clear
B)
contractility
done
clear
C)
rhythmicity
done
clear
D)
toxicity
done
clear
View Answer play_arrow
question_answer 148) In human beings, which hormone acts as a mild growth hormone?
A)
Prolactin
done
clear
B)
Oestrogen
done
clear
C)
Progesterone
done
clear
D)
Cortisol
done
clear
View Answer play_arrow
question_answer 149) Which one is not diploblastic?
A)
Sponge
done
clear
B)
Cnidarian
done
clear
C)
Nematoda
done
clear
D)
Tenophora
done
clear
View Answer play_arrow
question_answer 150) On the basis of utility, Nagpuri buffaloes are categorized as
A)
milkers
done
clear
B)
dual purpose
done
clear
C)
drought cattle
done
clear
D)
grazers
done
clear
View Answer play_arrow
question_answer 151) In algae, the bacteriological filter is
A)
Oscillatoria
done
clear
B)
Bafrachospermum
done
clear
C)
Nostoc
done
clear
D)
Cosmarium
done
clear
View Answer play_arrow
question_answer 152) This place in India is called The Golden Mine of Liverworts'
A)
Eastern Himalayas
done
clear
B)
Western Himalayas
done
clear
C)
Western Ghats
done
clear
D)
Eastern Ghats
done
clear
View Answer play_arrow
question_answer 153) Which one of the following pairs of plants are not seed producers?
A)
Fem and Funaria
done
clear
B)
Funaria and Ficus
done
clear
C)
Ficus and Pinus
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 154) What type of sorus is present in Nephrolepsis?
A)
Coenosorus
done
clear
B)
Simple
done
clear
C)
Mixed
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 155) In Pinus
A)
seed is winged but pollen grains are not
done
clear
B)
both seed and pollen grains are wingless
done
clear
C)
pollen grains are winged but seed is not
done
clear
D)
both seeds and pollen grains are winged
done
clear
View Answer play_arrow
question_answer 156) One of the set is wrongly matched
A)
Chrysan them urn - Sucker
done
clear
B)
Garlic - Cloves
done
clear
C)
Cyanodon - Stolen
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 157) Conn is an
A)
underground vertical leaf
done
clear
B)
underground swollen stem
done
clear
C)
underground horizontal root
done
clear
D)
underground horizontal leaf
done
clear
View Answer play_arrow
question_answer 158) Which of the following plants are all roots?
A)
Podostemon
done
clear
B)
Lemna
done
clear
C)
Wolffia
done
clear
D)
Urricularia
done
clear
View Answer play_arrow
question_answer 159) The petiole modified into flattened lamina like structure is present in
A)
Acacia nilotica
done
clear
B)
Acacia Arabica
done
clear
C)
Acacia Australasia
done
clear
D)
Acacia asiriculo form is
done
clear
View Answer play_arrow
question_answer 160) In Gloriosa, the tendrillar part is formed by
A)
stipule
done
clear
B)
leaf apex
done
clear
C)
leaf petiole
done
clear
D)
axillary bud
done
clear
View Answer play_arrow
question_answer 161) Feathery stigma belongs to
A)
Wheat
done
clear
B)
pea
done
clear
C)
Datura
done
clear
D)
Caesalpinia
done
clear
View Answer play_arrow
question_answer 162) Diadelphy can be found in
A)
Pisum
done
clear
B)
Citrus
done
clear
C)
Bombax
done
clear
D)
Brassica
done
clear
View Answer play_arrow
question_answer 163) One of the following is not a flower
A)
Shoe flower
done
clear
B)
Passion flower
done
clear
C)
Sunflower
done
clear
D)
May flower
done
clear
View Answer play_arrow
question_answer 164) Catkin inflorescence is characteristic of
A)
Morus
done
clear
B)
Triticum
done
clear
C)
Ficus
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 165) Fruit of Hibiscus esculentus is
A)
capsule
done
clear
B)
cypsela
done
clear
C)
legume
done
clear
D)
pome
done
clear
View Answer play_arrow
question_answer 166) The naked seeded plants are the
A)
gymnosperms
done
clear
B)
monocots
done
clear
C)
dicots
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 167) Pollinia are found in
A)
Rubiaceae
done
clear
B)
Asteraceae
done
clear
C)
Asclepiadaceae
done
clear
D)
Myrtaceae
done
clear
View Answer play_arrow
question_answer 168) Wood commonly used for making cricket bat is:
A)
Cedrus
done
clear
B)
Salix
done
clear
C)
Tecrona
done
clear
D)
Morus
done
clear
View Answer play_arrow
question_answer 169) Enucleated cells at maturity are
A)
palisade cells
done
clear
B)
guard cells
done
clear
C)
companion cells
done
clear
D)
sieve elements
done
clear
View Answer play_arrow
question_answer 170) Sunken stomata are present in
A)
mesophyte
done
clear
B)
xerophyte
done
clear
C)
epiphytes
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 171) Atactostele vascular bundles are
A)
scattered in the ground tissue
done
clear
B)
three in number
done
clear
C)
in form of ring around
done
clear
D)
broken vascular bundles
done
clear
View Answer play_arrow
question_answer 172) Pure line breed refers to
A)
homozygosity
done
clear
B)
heterozygosity
done
clear
C)
homozygosity with only dominant genes
done
clear
D)
heterozygosity and linkage
done
clear
View Answer play_arrow
question_answer 173) Chlorophyll-a is characterized by the side group
A)
methyl
done
clear
B)
aldehyde
done
clear
C)
phytol
done
clear
D)
ketone
done
clear
View Answer play_arrow
question_answer 174) Which of the following plant is efficient converter of solar energy whose net productivity is \[2-4\text{ }kg/{{m}^{2}}/yr\]or even higher?
A)
Wheat
done
clear
B)
Rice
done
clear
C)
Sugarcane
done
clear
D)
Bajra
done
clear
View Answer play_arrow
question_answer 175) Chloroplast has maximum quantity of .......... in stroma.
A)
dehydrogenase
done
clear
B)
RuDP carboxylase
done
clear
C)
pyruvic carboxylase
done
clear
D)
hexokinase
done
clear
View Answer play_arrow
question_answer 176) Photorespiration takes place in plants where carbon fixation occurs through
A)
Calvin cycle
done
clear
B)
Hatch and Slack cycle
done
clear
C)
glycolysis
done
clear
D)
Krebs' cycle
done
clear
View Answer play_arrow
question_answer 177) When an ovary develops into a fruit without fertilization it is called
A)
porogamy
done
clear
B)
apospory
done
clear
C)
apogamy
done
clear
D)
parthenocarpy
done
clear
View Answer play_arrow
question_answer 178) Vivipary is shown by
A)
Cocos
done
clear
B)
Squash
done
clear
C)
Rhizophora
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 179) GA usually promotes
A)
sterility in flowers
done
clear
B)
maleness in flowers
done
clear
C)
femaleness in flowers
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 180) Ethylene is
A)
gaseous hormone
done
clear
B)
largest hormone
done
clear
C)
liquid hormone
done
clear
D)
solid hormone
done
clear
View Answer play_arrow
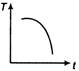
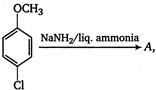 the major product A is
the major product A is
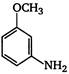
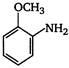
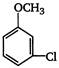
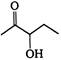
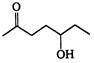
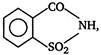 the given structural formula is of
the given structural formula is of
