question_answer 1) A tuning fork produce 2 beats when sounded with one oscillator of frequency 514 Hz, and produces 6 beats with the other oscillator of frequency 510 Hz. The frequency of tuning fork is
A)
516 Hz
done
clear
B)
510 Hz
done
clear
C)
514 Hz
done
clear
D)
520 Hz
done
clear
View Answer play_arrow
question_answer 2) Wavelength of the particle whose momentum is P
A)
\[\frac{h}{p}\]
done
clear
B)
\[hp\]
done
clear
C)
\[\frac{p}{h}\]
done
clear
D)
\[p+h\]
done
clear
View Answer play_arrow
question_answer 3) Colour of a star indicates its
A)
density
done
clear
B)
distance
done
clear
C)
energy
done
clear
D)
temperature
done
clear
View Answer play_arrow
question_answer 4) Which of the following has same dimensions to that of Plancks constant?
A)
Work
done
clear
B)
Energy
done
clear
C)
Linear momentum
done
clear
D)
Angular momentum
done
clear
View Answer play_arrow
question_answer 5) Temperature of a piece of silicon is raised from\[27{}^\circ C\]to\[100{}^\circ C,\]then its conductivity
A)
increases
done
clear
B)
decreases
done
clear
C)
remains same
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 6) If a liquid does not wet a solid surface, then its angle of contact is
A)
acute angle
done
clear
B)
obtuse angle
done
clear
C)
right angle
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 7) Three capacitors of 3, 2 and\[6\mu F\]are connected in series with a battery of 10 V. Then charge on\[3\mu F\]capacitor will be
A)
\[10\mu C\]
done
clear
B)
\[12\mu C\]
done
clear
C)
\[14\mu C\]
done
clear
D)
\[5\mu C\]
done
clear
View Answer play_arrow
question_answer 8) To hear the echo in 1 s, the minimum distance of the source from the reflecting surface should be
A)
28m
done
clear
B)
18m
done
clear
C)
19m
done
clear
D)
165m
done
clear
View Answer play_arrow
question_answer 9) If length of a simple pendulum increases by 300%, then its time period increases by
A)
100%
done
clear
B)
200%
done
clear
C)
300%
done
clear
D)
400%
done
clear
View Answer play_arrow
question_answer 10) Half-life of radium is 1600 yr, then its 1/16th part will remain undecayed after
A)
1200 yr
done
clear
B)
6400 yr
done
clear
C)
1800 yr
done
clear
D)
2200 yr
done
clear
View Answer play_arrow
question_answer 11) When a light beam enters into water from air, then which of the following does not change?
A)
Velocity
done
clear
B)
Frequency
done
clear
C)
Wavelength
done
clear
D)
Amplitude
done
clear
View Answer play_arrow
question_answer 12) Sound waves produced in gas are
A)
longitudinal
done
clear
B)
transversal
done
clear
C)
stationary
done
clear
D)
progressive
done
clear
View Answer play_arrow
question_answer 13) When a force F is applied on a wire of length L and radius r, extension produce is\[l\]. If same force F is applied on the same material wire of length 2 L and radius 2r, then extension will be
A)
\[l\]
done
clear
B)
\[2l\]
done
clear
C)
\[l/2\]
done
clear
D)
\[4l\]
done
clear
View Answer play_arrow
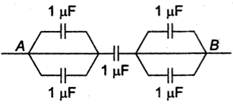
question_answer 14)
Equivalent capacity between A and B is
A)
\[2\mu F\]
done
clear
B)
\[3\mu F\]
done
clear
C)
\[4\mu F\]
done
clear
D)
\[0.5\mu F\]
done
clear
View Answer play_arrow
question_answer 15) Angular velocity of second hand of a clock is
A)
\[\frac{\pi }{30}rad/s\]
done
clear
B)
\[\frac{\pi }{60}rad/s\]
done
clear
C)
\[\frac{\pi }{15}rad/s\]
done
clear
D)
\[\frac{\pi }{2}rad/s\]
done
clear
View Answer play_arrow
question_answer 16) n-type semiconductor is
A)
positive charged
done
clear
B)
neutral
done
clear
C)
negatively charged
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 17) Intensity of a source of a 100 cd at a distance 2 m is
A)
25 lux
done
clear
B)
50 lux
done
clear
C)
75 lux
done
clear
D)
150 lux
done
clear
View Answer play_arrow
question_answer 18) Atomic number of an atom is Z and mass number is m, then number of neutrons in its nucleus is
A)
\[m-Z\]
done
clear
B)
\[\frac{m}{Z}\]
done
clear
C)
\[m+Z\]
done
clear
D)
\[m\times Z\]
done
clear
View Answer play_arrow
question_answer 19) Power factor of L-CR circuit in resonation condition is
A)
1
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
zero
done
clear
D)
\[\frac{1}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 20) If tension of sonometer wire is made four times, then its frequency will change by a factor of
A)
2
done
clear
B)
4
done
clear
C)
1/2
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 21) Which of the following has maximum specific heat?
A)
Water
done
clear
B)
Alcohol
done
clear
C)
Glycerene
done
clear
D)
Oil
done
clear
View Answer play_arrow
question_answer 22) Momentum of a body of mass 1 kg is 10 kg-m/s, then its kinetic energy will be
A)
100 J
done
clear
B)
50 J
done
clear
C)
1000 J
done
clear
D)
200 J
done
clear
View Answer play_arrow
question_answer 23) Nucleus of an atom has
A)
\[e\]and\[n\]
done
clear
B)
\[e\]and\[p\]
done
clear
C)
\[p\]and\[n\]
done
clear
D)
\[e,p\]and\[n\]
done
clear
View Answer play_arrow
question_answer 24) Which of the following is scalar quantity?
A)
Current
done
clear
B)
Velocity
done
clear
C)
Force
done
clear
D)
Acceleration
done
clear
View Answer play_arrow
question_answer 25) Equation of a progressive sound wave is\[y=a\sin \left( 400\pi t-\frac{\pi x}{0.85} \right)\]where\[x\]in (metre)\[t\] (second), then frequency of wave is
A)
200 Hz
done
clear
B)
400 Hz
done
clear
C)
500 Hz
done
clear
D)
600 Hz
done
clear
View Answer play_arrow
question_answer 26) Wavelength of the wave in the above question is
A)
1.7m
done
clear
B)
8.5m
done
clear
C)
0.85m
done
clear
D)
0.17m
done
clear
View Answer play_arrow
question_answer 27) A wheel completes 2000 turns in completing a distance of 9.5 km, then diameter of the wheel is
A)
1.5m
done
clear
B)
1.5cm
done
clear
C)
7.5m
done
clear
D)
7.5cm
done
clear
View Answer play_arrow
question_answer 28) In nth orbit of hydrogen atom energy \[E=\frac{-13.6}{{{n}^{2}}}eV,\]then energy required to displace electrons from\[n=1\]to\[n=2\]is
A)
\[-10.2\text{ }eV\]
done
clear
B)
3.4 eV
done
clear
C)
\[-13.6\text{ }eV\]
done
clear
D)
1.51 eV
done
clear
View Answer play_arrow
question_answer 29) Relation of wavelengths of sound and light is
A)
\[{{\lambda }_{S}}<{{\lambda }_{L}}\]
done
clear
B)
\[{{\lambda }_{S}}>{{\lambda }_{L}}\]
done
clear
C)
\[{{\lambda }_{S}}={{\lambda }_{L}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 30) In Youngs experiment, if separation between slits is made half, then fringe width will become
A)
double
done
clear
B)
four times
done
clear
C)
half
done
clear
D)
remains same
done
clear
View Answer play_arrow
question_answer 31) Resolving power of human eye is
A)
\[0.1\,\,\]
done
clear
B)
\[1\,\]
done
clear
C)
\[0.1\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 32) Dancing of small pieces of camphor on the surface of water is due to
A)
viscosity
done
clear
B)
surface tension
done
clear
C)
weight
done
clear
D)
lifting force
done
clear
View Answer play_arrow
question_answer 33) Relation between specific heats of an ideal gas
A)
\[{{C}_{P}}+{{C}_{V}}=R\]
done
clear
B)
\[{{C}_{P}}-{{C}_{V}}=R\]
done
clear
C)
\[\frac{{{C}_{P}}}{{{C}_{V}}}=R\]
done
clear
D)
\[\frac{{{C}_{V}}}{{{C}_{P}}}=R\]
done
clear
View Answer play_arrow
question_answer 34) Who gave the kinetic molecular theory of gas?
A)
Device
done
clear
B)
Einstein
done
clear
C)
Newton
done
clear
D)
Bernoulli
done
clear
View Answer play_arrow
question_answer 35) Potential energy of a particle at a distance\[x\]from mean position, which is executing simple hormonic motion
A)
\[\frac{1}{2}m{{\omega }^{2}}{{x}^{2}}\]
done
clear
B)
zero
done
clear
C)
\[{{m}^{2}}\omega x\]
done
clear
D)
\[\frac{1}{2}{{m}^{2}}{{\omega }^{2}}{{x}^{2}}\]
done
clear
View Answer play_arrow
question_answer 36) If distance between light source and screen is doubled, then intensity of light will become
A)
half
done
clear
B)
double
done
clear
C)
remains same
done
clear
D)
1/4th
done
clear
View Answer play_arrow
question_answer 37) The colour of a red doth in green light appears
A)
red
done
clear
B)
green
done
clear
C)
orange
done
clear
D)
black
done
clear
View Answer play_arrow
question_answer 38) An inductor and a capacitor are connected in an AC circuit inductance and capacitance are 1 H and\[25\mu F,\]then for maximum current, angular frequency will be (circuit is connected in series)
A)
200 rad/s
done
clear
B)
50 rad/s
done
clear
C)
100 rad/s
done
clear
D)
150 rad/s
done
clear
View Answer play_arrow
question_answer 39) A satellite of mass m, revolving round a planet of mass Min a circular orbit of radius r, then orbital velocity of the satellite is
A)
\[\frac{mg}{r}\]
done
clear
B)
\[\sqrt{\frac{GM}{r}}\]
done
clear
C)
\[\sqrt{\frac{2GM}{r}}\]
done
clear
D)
\[\frac{GM}{r}\]
done
clear
View Answer play_arrow
question_answer 40) Energy stored in the unit volume of a wire due to its elasticity is
A)
\[\frac{1}{2}(force\times strain)\]
done
clear
B)
\[\frac{1}{2}stress\times strain\]
done
clear
C)
stress/strain
done
clear
D)
force/strain
done
clear
View Answer play_arrow
question_answer 41) Value of\[-40{}^\circ C\]in Fahrenheit scale is
A)
\[-40F\]
done
clear
B)
32 F
done
clear
C)
\[-32\text{ }F\]
done
clear
D)
\[40{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 42) Spectrum of a star is shifting towards violet colour, then the star
A)
is going away from earth
done
clear
B)
is coming towards earth
done
clear
C)
intensity of light of star is increasing
done
clear
D)
intensity of light of star is decreasing
done
clear
View Answer play_arrow
question_answer 43) The amount of heat required to change 1 g \[(0{}^\circ C)\]of ice into water of\[100{}^\circ C,\]is
A)
716 cal
done
clear
B)
500 cal
done
clear
C)
180 cal
done
clear
D)
100 cal
done
clear
View Answer play_arrow
question_answer 44) Susceptibility of a magnetic field is
A)
\[\chi =\frac{l}{H}\]
done
clear
B)
\[\chi =\frac{B}{H}\]
done
clear
C)
\[\chi =\frac{M}{V}\]
done
clear
D)
\[\chi =\frac{M}{H}\]
done
clear
View Answer play_arrow
question_answer 45) Sphere, disc and ring are allowed to roll down on an inclined plane from its top, then order in which they reach at the bottom will be
A)
ring, disc, sphere
done
clear
B)
sphere, disc, ring
done
clear
C)
disc, ring, sphere
done
clear
D)
sphere, ring, disc
done
clear
View Answer play_arrow
question_answer 46) If dopping in the P region is high, then N region
A)
depletion layer will. more towards P
done
clear
B)
depletion layer will more towards N
done
clear
C)
depletion layer will remain unchanged
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 47) Work of invertor is
A)
to change AC into DC
done
clear
B)
to change DC into AC
done
clear
C)
to regulate voltage
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 48) Dimensions of torque is
A)
\[[{{M}^{1}}{{L}^{2}}{{T}^{-2}}]\]
done
clear
B)
\[[{{M}^{2}}{{L}^{2}}{{T}^{2}}]\]
done
clear
C)
\[[{{M}^{-1}}L{{T}^{-1}}]\]
done
clear
D)
\[[{{M}^{-2}}{{L}^{-2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 49) For a triode valve\[\mu =50,\Delta {{V}_{g}}=0.2V,\]then value of\[\Delta {{V}_{p}}\]is
A)
5V
done
clear
B)
10V
done
clear
C)
0.2V
done
clear
D)
50V
done
clear
View Answer play_arrow
question_answer 50) Which of the following has maximum property of elasticity?
A)
Rubber
done
clear
B)
Lead
done
clear
C)
Wood
done
clear
D)
Steel
done
clear
View Answer play_arrow
question_answer 51) If we made half the radius of earth, then duration of day will become
A)
6 h
done
clear
B)
12 h
done
clear
C)
24 h
done
clear
D)
3 h
done
clear
View Answer play_arrow
question_answer 52) A stone is dropped in 19.6 m depth well, echo is heard in 2.06 s, then velocity of sound is
A)
315.2 m/s
done
clear
B)
320.5 m/s
done
clear
C)
326.7 m/s
done
clear
D)
332.4 m/s
done
clear
View Answer play_arrow
question_answer 53) An electron is moving with velocity v in the direction of magnetic field B, then force acting on electron is
A)
zero
done
clear
B)
\[e(v\times B)\]
done
clear
C)
\[e(B\times v)\]
done
clear
D)
200 J
done
clear
View Answer play_arrow
question_answer 54) If temperature of an object is\[140{}^\circ F,\]then its temperature in centrigrade is
A)
\[105{}^\circ C\]
done
clear
B)
\[32{}^\circ C\]
done
clear
C)
\[140{}^\circ C\]
done
clear
D)
\[60{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 55) Focal length of a lens is + 10 cm and its refractive index is 1.4, it is dipped in a liquid of refractive index 1.6, then its focal length becomes
A)
\[-16\text{ }cm\]
done
clear
B)
\[+32\text{ }cm\]
done
clear
C)
\[+16\text{ }cm\]
done
clear
D)
\[-32cm\]
done
clear
View Answer play_arrow
question_answer 56) Ten wires each of resistance\[1\,\Omega \] are connected in parallel, then total resistance will be
A)
\[10\,\,\Omega \]
done
clear
B)
\[1\,\,\Omega \]
done
clear
C)
\[0.1\,\,\Omega \]
done
clear
D)
\[0.001\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 57) If focal length of convex lens is 9 cm and that of a concave lens is\[-18\text{ }cm,\]then focal length of combination is
A)
\[+9\text{ }cm\]
done
clear
B)
\[-18\text{ }cm\]
done
clear
C)
\[-9cm\]
done
clear
D)
\[+18cm\]
done
clear
View Answer play_arrow
question_answer 58) Two mirror are put at an angle of\[60{}^\circ C,\]then number of image of an object placed between the mirrors is
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 59) An observer is coming towards plane mirror with a speed of 6 m/s, then apparent velocity of his image is
A)
6 m/s
done
clear
B)
4 m/s
done
clear
C)
12 m/s
done
clear
D)
3 m/s
done
clear
View Answer play_arrow
question_answer 60) Speed of light in vacuum is
A)
\[\frac{1}{\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}}\]
done
clear
B)
\[\sqrt{\frac{{{\mu }_{0}}}{{{\varepsilon }_{0}}}}\]
done
clear
C)
\[\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}\]
done
clear
D)
\[\sqrt{\frac{{{\varepsilon }_{0}}}{{{\mu }_{0}}}}\]
done
clear
View Answer play_arrow
question_answer 61) Fermentation is
A)
reversible
done
clear
B)
exothermic
done
clear
C)
endothermic
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 62) The ionisation percentage of\[0.2\text{ }M\text{ }HCN\]the solution is 0.02%. What will be the value of ionisation constant?
A)
\[8\times {{10}^{-5}}\]
done
clear
B)
\[8\times {{10}^{-9}}\]
done
clear
C)
\[8\times {{10}^{-7}}\]
done
clear
D)
\[8\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 63) Which one act as Bronsted acid as well as Bronsted base?
A)
\[C{{l}^{-}}\]
done
clear
B)
\[O{{H}^{-}}\]
done
clear
C)
\[HCl\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 64) If the pH of\[HCl\]soltion is 2 then the molar concentration of this will be
A)
0.01 M
done
clear
B)
2 M
done
clear
C)
0.4 M
done
clear
D)
0.02 M
done
clear
View Answer play_arrow
question_answer 65) \[B{{F}_{3}}\]molecule is
A)
Lewis base
done
clear
B)
Lewis acid
done
clear
C)
Neutral
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 66) Which salt is insoluble in water?
A)
\[N{{H}_{4}}Cl\]
done
clear
B)
\[KCl\]
done
clear
C)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
D)
\[NaCl\]
done
clear
View Answer play_arrow
question_answer 67) Which compound forms urotropine when reacts with\[N{{H}_{3}}\]?
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 68) The compound formed by the ozonolysis of acetylene
A)
glycol
done
clear
B)
acetic acid
done
clear
C)
ethylene ozonide
done
clear
D)
glyoxal
done
clear
View Answer play_arrow
question_answer 69) Number of electron in one molecule of\[N{{H}_{3}}\]
A)
17
done
clear
B)
34
done
clear
C)
7
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 70) The value of magnetic quantum number for\[l=3\]
A)
\[0,+1,+2,+3\]
done
clear
B)
\[0,\pm 1,\pm 2,\pm 3\]
done
clear
C)
\[-1,-2,-3\]
done
clear
D)
\[\pm 1,\pm 2,\pm 3\]
done
clear
View Answer play_arrow
question_answer 71) In the presence of ether, 2-choloropropane reacts with sodium and forms
A)
n-hexane
done
clear
B)
2,3-dimethyl butane
done
clear
C)
n-hexene
done
clear
D)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 72) The geometry of ammonia is
A)
tetrahedral
done
clear
B)
pyramidal
done
clear
C)
triangular
done
clear
D)
trigonal bipyramidal
done
clear
View Answer play_arrow
question_answer 73) Which one is colourless?
A)
\[C{{o}^{2+}}\]
done
clear
B)
\[N{{i}^{2+}}\]
done
clear
C)
\[F{{e}^{3+}}\]
done
clear
D)
\[C{{u}^{+}}\]
done
clear
View Answer play_arrow
question_answer 74) Which one have the highest melting point?
A)
\[CsCl\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[He\]
done
clear
D)
\[CHC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 75) Which one will not show Cannizzaro reaction?
A)
Trimethyl acetaldehyde
done
clear
B)
Formaldehyde
done
clear
C)
Acetaldehyde
done
clear
D)
Benzaldehyde
done
clear
View Answer play_arrow
question_answer 76) When 23 g sodium metal reacts with methyl alcohol, it will form
A)
1 moles of \[{{H}_{2}}\]
done
clear
B)
2 mole of \[{{H}_{2}}\]
done
clear
C)
\[\frac{1}{2}\]mole of \[{{H}_{2}}\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 77) Which inorganic compound on heating changes into organic compound?
A)
Ammonium cyanide
done
clear
B)
Sodalime
done
clear
C)
Potassium cyanide
done
clear
D)
Sodamide
done
clear
View Answer play_arrow
question_answer 78) Which of the following compound will not show aldol condensation?
A)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
View Answer play_arrow
question_answer 79) In first transition series, which element show maximum oxidation states
A)
\[Co\]
done
clear
B)
\[Mn\]
done
clear
C)
\[Cr\]
done
clear
D)
\[Fe\]
done
clear
View Answer play_arrow
question_answer 80) The bad odour compound formed by the reaction between chloroform and aniline in the presence of alcoholic KOH
A)
acetylene
done
clear
B)
nitro benzene
done
clear
C)
phenyl isocyanate
done
clear
D)
chloromine
done
clear
View Answer play_arrow
question_answer 81) \[N{{O}_{2}}+\frac{1}{2}{{O}_{2}}N{{O}_{3}}........{{K}_{1}}\] \[2N{{O}_{2}}+{{O}_{2}}2N{{O}_{3}}........{{K}_{2}}\] For the above reaction the relation between equilibrium constant is
A)
\[{{K}_{2}}={{({{K}_{1}})}^{2}}\]
done
clear
B)
\[{{K}_{2}}=\frac{1}{{{K}_{1}}}\]
done
clear
C)
\[{{K}_{2}}=\frac{1}{K_{1}^{2}}\]
done
clear
D)
\[{{K}_{1}}={{K}_{2}}\]
done
clear
View Answer play_arrow
question_answer 82) The condensation product of reaction between phenol and phthalic anhydride
A)
methyl orange
done
clear
B)
salicylic acid
done
clear
C)
phenol red
done
clear
D)
phenolphthalein
done
clear
View Answer play_arrow
question_answer 83) Which characteristic property is not shown by carboxylic acid group?
A)
Alkly group
done
clear
B)
-COOH group
done
clear
C)
group
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 84) The product of reaction between chloroform and acetone is used as
A)
insecticide
done
clear
B)
analgesic
done
clear
C)
hypnotic
done
clear
D)
war gas
done
clear
View Answer play_arrow
question_answer 85) The strongest Bronsted base is
A)
\[HClO_{2}^{-}\]
done
clear
B)
\[HCl{{O}^{-}}\]
done
clear
C)
\[HClO_{3}^{-}\]
done
clear
D)
\[HCl{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 86) The compound which have conjugate double bond
A)
butyne
done
clear
B)
butylene
done
clear
C)
isobutylene
done
clear
D)
butadiene
done
clear
View Answer play_arrow
question_answer 87) Which salt on heating, does not give brown gas on heating?
A)
\[LiN{{O}_{3}}\]
done
clear
B)
\[AgN{{O}_{3}}\]
done
clear
C)
\[Pb{{(N{{O}_{3}})}_{2}}\]
done
clear
D)
\[KN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 88) The product formed by the reaction between acetylene and\[HOCl\]
A)
ethylene chloride
done
clear
B)
vinyl chloride
done
clear
C)
dichloro acetaldehyde
done
clear
D)
ethyledene chloride
done
clear
View Answer play_arrow
question_answer 89) The characteristic ion of an acid in an aqueous solution is
A)
\[{{H}^{+}}\]
done
clear
B)
\[H_{2}^{+}\]
done
clear
C)
\[{{H}_{3}}{{O}^{+}}\]
done
clear
D)
\[{{H}_{4}}{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 90) The most paramagnetic is
A)
\[C{{r}^{3+}}\]
done
clear
B)
\[C{{O}^{2+}}\]
done
clear
C)
\[M{{n}^{2+}}\]
done
clear
D)
\[F{{e}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 91) The present undesirable matrix in mineral is called as
A)
flux
done
clear
B)
slag
done
clear
C)
gangue
done
clear
D)
alloy
done
clear
View Answer play_arrow
question_answer 92) The compound with maximum covalent character is
A)
\[AlC{{l}_{3}}\]
done
clear
B)
\[Al{{I}_{3}}\]
done
clear
C)
\[Ag{{I}_{2}}\]
done
clear
D)
\[NaI\]
done
clear
View Answer play_arrow
question_answer 93) \[N{{F}_{3}}\]and\[B{{F}_{3}}\]both are covalent, but\[B{{F}_{3}}\]is non-polar, while\[N{{F}_{3}}\]is polar because
A)
boron is non-metal in free state, while\[{{N}_{2}}\]is gas
done
clear
B)
there is no dipole moment in\[B-F\]bond while\[N-F\]bond have some dipole moment
done
clear
C)
\[B{{F}_{3}}\]is planar while\[N{{F}_{3}}\]is pyramidal
done
clear
D)
boron atom is smaller than nitrogen atom
done
clear
View Answer play_arrow
question_answer 94) Many compounds are formed by carbon atom because of its
A)
more reactivity
done
clear
B)
covalent and ionic nature
done
clear
C)
variable valency
done
clear
D)
catenation nature
done
clear
View Answer play_arrow
question_answer 95) For the complete combustion of 4 L ethane, how much oxygen is required?
A)
14 L
done
clear
B)
4L
done
clear
C)
8L
done
clear
D)
12 L
done
clear
View Answer play_arrow
question_answer 96) In a group of Periodic Table what is the basis of
A)
ionic potential
done
clear
B)
electron affinity
done
clear
C)
electron negativity
done
clear
D)
number of covalent electron
done
clear
View Answer play_arrow
question_answer 97) The work of cone.\[{{H}_{2}}S{{O}_{4}}\]in esterification process is as
A)
dehydrating agent and catalyst
done
clear
B)
dehydrating agent
done
clear
C)
hydrolysing agent
done
clear
D)
catalyst
done
clear
View Answer play_arrow
question_answer 98) When magnesium is heated, the another compound formed with\[MgO\]is
A)
\[M{{g}_{3}}{{N}_{2}}\]
done
clear
B)
\[Mg{{(N{{O}_{3}})}_{2}}\]
done
clear
C)
\[MgC{{O}_{3}}\]
done
clear
D)
\[Mg{{(N{{O}_{3}})}_{3}}\]
done
clear
View Answer play_arrow
question_answer 99) The\[C-H\]bond energy in ethane, ethene and ethyne
A)
more in ethane
done
clear
B)
more in ethane
done
clear
C)
more in ethyne
done
clear
D)
same in all these
done
clear
View Answer play_arrow
question_answer 100) The solubility of\[AgCl\]is 1.435 g/L What will be the solubility product?
A)
\[{{10}^{-4}}\]
done
clear
B)
0.01
done
clear
C)
0.001
done
clear
D)
0.1
done
clear
View Answer play_arrow
question_answer 101) If two elements X and Y have 3 and 6 electrons in its outermost shell, then the molecular formula of compound formed by the combination of X and Y
A)
\[XY\]
done
clear
B)
\[{{X}_{3}}Y\]
done
clear
C)
\[{{X}_{2}}Y\]
done
clear
D)
\[{{X}_{2}}{{Y}_{3}}\]
done
clear
View Answer play_arrow
question_answer 102) The IUPAC name of\[\underset{C{{H}_{2}}.}{\overset{OH}{\mathop{|}}}\,\underset{C{{H}_{2}}.}{\overset{OH}{\mathop{|}}}\,\underset{C{{H}_{2}}.}{\overset{OH}{\mathop{|}}}\,\]is
A)
1,2,3-propane tri-ol
done
clear
B)
3-hydroxy butanoic acid
done
clear
C)
1,2-ethane di-ol
done
clear
D)
3-methyl butanol-1
done
clear
View Answer play_arrow
question_answer 103) The number of\[{{H}^{+}}\]ion in half normal solution of\[N{{a}_{2}}C{{O}_{3}}\]and\[C{{H}_{3}}COOH\]
A)
\[>{{10}^{-7}}m\]
done
clear
B)
\[{{10}^{-7}}m\]
done
clear
C)
\[<{{10}^{-7}}m\]
done
clear
D)
\[{{10}^{-5}}m\]
done
clear
View Answer play_arrow
question_answer 104) Which of the following is not a redox reaction?
A)
\[KCN+Fe{{(CN)}_{2}}\xrightarrow[{}]{{}}{{K}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
B)
\[Rb+{{H}_{2}}O\xrightarrow[{}]{{}}RbOH+{{H}_{2}}\]
done
clear
C)
\[{{H}_{2}}{{O}_{2}}\xrightarrow[{}]{{}}{{H}_{2}}O+O\]
done
clear
D)
\[Cu{{I}_{2}}\xrightarrow[{}]{{}}CuI+{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 105) The correct electronic configuration of element with atomic number 29.
A)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}},4{{s}^{1}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{17}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 106) Which of the following statement is correct for ketone and acetaldehyde?
A)
Both reacts with HCN and forms hydrine
done
clear
B)
Both reacts with\[NaOH\]and forms polymer
done
clear
C)
both forms add on oxidation and reduction
done
clear
D)
Both forms alcohol on reduction and oxidation
done
clear
View Answer play_arrow
question_answer 107) The prefix word alkali for alkaline metals shows
A)
lustre of silver
done
clear
B)
ashes of plant
done
clear
C)
metallic nature
done
clear
D)
active metal
done
clear
View Answer play_arrow
question_answer 108) \[2X+2Y\xrightarrow[{}]{{}}2Z\] The equilibrium constant for the above reaction
A)
\[\frac{[2X][2Y]}{[2Z]}\]
done
clear
B)
\[\frac{[X][Y]}{[Z]}\]
done
clear
C)
\[\frac{{{[Z]}^{2}}}{{{[X]}^{2}}{{[Y]}^{2}}}\]
done
clear
D)
\[\frac{{{[Z]}^{2}}}{[X][Y]}\]
done
clear
View Answer play_arrow
question_answer 109) The solubility product for XY is
A)
\[{{s}^{2}}\]
done
clear
B)
\[2s\]
done
clear
C)
\[4{{s}^{2}}\]
done
clear
D)
\[2s\]
done
clear
View Answer play_arrow
question_answer 110) Aldehyde and ketone can be differentiated by
A)
sodium bi sulphite
done
clear
B)
Fehling solution
done
clear
C)
ammonia
done
clear
D)
sulphuric acid
done
clear
View Answer play_arrow
question_answer 111) The product of the reaction between chloral and\[NaOH\]is
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 112) The correct reactivity older is
A)
\[C{{H}_{3}}CHO>{{(C{{H}_{3}})}_{2}}CO>C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CO>C{{H}_{3}}CHO>C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}\]
done
clear
C)
\[C{{H}_{3}}CHO>C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}>{{(C{{H}_{3}})}_{2}}CO\]
done
clear
D)
\[C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}>C{{H}_{3}}CHO>{{(C{{H}_{3}})}_{2}}CO\]
done
clear
View Answer play_arrow
question_answer 113) The example of condensation polymerization is
A)
formaldehyde - metaformaldehyde
done
clear
B)
acetaldehyde-para acetaldehyde
done
clear
C)
acetone - mesityl oxide
done
clear
D)
ethene-polyethene
done
clear
View Answer play_arrow
question_answer 114) The probability of finding of three unpaired electron in nitrogen atom is defined by
A)
Aufbaus principle
done
clear
B)
Uncertainty principle
done
clear
C)
Faults principle
done
clear
D)
Hunds rule
done
clear
View Answer play_arrow
question_answer 115) In a reversible reaction if the concentration of reactants and products are doubled, the value of\[{{K}_{c}}\]will be
A)
half of the initial value
done
clear
B)
double of initial value
done
clear
C)
one fourth of the initial value
done
clear
D)
same the initial value
done
clear
View Answer play_arrow
question_answer 116) The main product of heating of sodium formate at 3600C is
A)
CO
done
clear
B)
sodium oxalate
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 117) The number of a (sigma) and\[\pi \](pi) bonds in 1-buene-3-yne is
A)
\[5\sigma ,5\pi \]
done
clear
B)
\[8\sigma ,6\pi \]
done
clear
C)
\[7\sigma ,3\pi \]
done
clear
D)
\[6\sigma ,4\pi \]
done
clear
View Answer play_arrow
question_answer 118) The correct size of iodine atom and iodine ion is
A)
\[{{I}^{-}}>{{I}^{+}}>I\]
done
clear
B)
\[I>{{I}^{+}}>{{I}^{-}}\]
done
clear
C)
\[I>{{I}^{-}}>{{I}^{+}}\]
done
clear
D)
\[{{I}^{+}}>I>{{I}^{-}}\]
done
clear
View Answer play_arrow
question_answer 119) The geemeticy of the compound of\[s{{p}^{3}}d\] hybridization
A)
pyramidal
done
clear
B)
triangular
done
clear
C)
planar
done
clear
D)
trigonal bipyramidal
done
clear
View Answer play_arrow
question_answer 120) Potassium nitrate is called
A)
Motifs salt
done
clear
B)
Chile salt petre
done
clear
C)
Indian salt petre
done
clear
D)
Gypsum
done
clear
View Answer play_arrow
question_answer 121) Operator genes are controls to which gene mechanism?
A)
Structural gene
done
clear
B)
Activator gene
done
clear
C)
Regulator gene
done
clear
D)
Modulac gene
done
clear
View Answer play_arrow
question_answer 122) How many ovum are developed in ovary of Hydra?
A)
Two
done
clear
B)
Many
done
clear
C)
One
done
clear
D)
Three
done
clear
View Answer play_arrow
question_answer 123) Respiration in Ascaris is
A)
aerobic respiration
done
clear
B)
anaerobic respiration
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 124) Type of blastula in chick is
A)
discoblastula
done
clear
B)
coeloblastula
done
clear
C)
amphiblastula
done
clear
D)
holbblastula
done
clear
View Answer play_arrow
question_answer 125) Respiration pigment of blood in cockroach is
A)
haemozoin
done
clear
B)
haemocyanin
done
clear
C)
haemoglobin
done
clear
D)
absent
done
clear
View Answer play_arrow
question_answer 126) Which organ is enlarged in malarial patient?
A)
Spleen
done
clear
B)
Kidney
done
clear
C)
Gall bladder
done
clear
D)
Liver
done
clear
View Answer play_arrow
question_answer 127) Golgi body is
A)
organ for protein synthesis
done
clear
B)
secretory organ
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 128) DNA is specific because it has
A)
number of nudeotides
done
clear
B)
specific nature of phosphate and sugar
done
clear
C)
arrangement of protein in DNA
done
clear
D)
specific nature of purine and pyrimidines
done
clear
View Answer play_arrow
question_answer 129) Role of carbohydrate in protoplasm
A)
as catalyst
done
clear
B)
for energy
done
clear
C)
as enzymes
done
clear
D)
for synthesis
done
clear
View Answer play_arrow
question_answer 130) In Amoeba hyaline cap is formed by
A)
around the food vacuole
done
clear
B)
around the contractile vacuole
done
clear
C)
around the nucleus
done
clear
D)
on pseudopodia
done
clear
View Answer play_arrow
question_answer 131) Which tissue covered with calcium phosphate?
A)
Bone
done
clear
B)
Muscles
done
clear
C)
Mesenchyma
done
clear
D)
Cartilage
done
clear
View Answer play_arrow
question_answer 132) Lampbrush chromosomes are present in
A)
Drosophilla
done
clear
B)
Ascaris
done
clear
C)
Hydra
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 133) Comb jelly is member of phylum
A)
Mollusca
done
clear
B)
Echinodermata
done
clear
C)
Coelenterata
done
clear
D)
Ctenophora
done
clear
View Answer play_arrow
question_answer 134) Jaws present in which of the following?
A)
Herdmania
done
clear
B)
Fishes
done
clear
C)
Petromyzon
done
clear
D)
Amphioxus
done
clear
View Answer play_arrow
question_answer 135) Which of the following is not insect?
A)
Spider
done
clear
B)
Grasshopper
done
clear
C)
Fly
done
clear
D)
Lepisma
done
clear
View Answer play_arrow
question_answer 136) Microlecithal eggs are present in
A)
Insects
done
clear
B)
Aves
done
clear
C)
Fishes
done
clear
D)
Mammals
done
clear
View Answer play_arrow
question_answer 137) Muscular movements of alimentarey canal are known as
A)
peristalsis
done
clear
B)
diastole
done
clear
C)
systole
done
clear
D)
muscles contraction
done
clear
View Answer play_arrow
question_answer 138) From acrosome, which secretes
A)
hyaluronic acid
done
clear
B)
hyaluronidase
done
clear
C)
TSH
done
clear
D)
fertilizing
done
clear
View Answer play_arrow
question_answer 139) When embryonic development is completed in mother but the embryo not accept nutritent from mother, which is known as
A)
ovoviparous
done
clear
B)
viviparous
done
clear
C)
oviparous
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 140) Gastrula is differ from blastula in
A)
three germ layers
done
clear
B)
micromeres
done
clear
C)
blastocoel
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 141) Which gland is worked opposite to pressure?
A)
Adrenal
done
clear
B)
parathyroid
done
clear
C)
Pineal
done
clear
D)
Thyroid
done
clear
View Answer play_arrow
question_answer 142) First heart sound is produced during closure of
A)
auriculo-ventricular valves
done
clear
B)
eustachian valve
done
clear
C)
sinus valve
done
clear
D)
seminular valves
done
clear
View Answer play_arrow
question_answer 143) In motor neuron the impulse following in which direction?
A)
In two direction
done
clear
B)
In one direction
done
clear
C)
In all direction
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 144) Nucleated RBCs are present in which
A)
Rabbit
done
clear
B)
Camel and llama
done
clear
C)
Embryo of human
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 145) Sertoli cells are associated with
A)
kidney of rabbit
done
clear
B)
ovary of frog
done
clear
C)
testis of rabbit
done
clear
D)
ovary of rabbit
done
clear
View Answer play_arrow
question_answer 146) Final excretion in urine of which substance is
A)
amino acid
done
clear
B)
urea
done
clear
C)
glucose and glycogen
done
clear
D)
uric acid
done
clear
View Answer play_arrow
question_answer 147) Haemoglobin in earthworm is present in
A)
RBC
done
clear
B)
protoplasm
done
clear
C)
coelomic fluid
done
clear
D)
plasma
done
clear
View Answer play_arrow
question_answer 148) Gametes formation in animals is found in
A)
ovaries
done
clear
B)
gonads
done
clear
C)
gall bladder
done
clear
D)
testes
done
clear
View Answer play_arrow
question_answer 149) Fertilization in earthworm is
A)
cross fertilization
done
clear
B)
combined fertilization
done
clear
C)
self-fertilization
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 150) Hydra is
A)
herbivorous
done
clear
B)
more developed
done
clear
C)
carnivorous
done
clear
D)
omnivorous
done
clear
View Answer play_arrow
question_answer 151) The pH of protoplasm is
A)
\[6.0\]
done
clear
B)
\[6.8\]
done
clear
C)
\[7.8\]
done
clear
D)
\[8.2\]
done
clear
View Answer play_arrow
question_answer 152) The maximum elements found in cytoplasm is
A)
\[C\]
done
clear
B)
\[He\]
done
clear
C)
\[H\]
done
clear
D)
\[N\]
done
clear
View Answer play_arrow
question_answer 153) The enzymes found in lysosomes are
A)
hydrolytic enzymes
done
clear
B)
proteases enzymes
done
clear
C)
Upases enzymes
done
clear
D)
cellulases enzymes
done
clear
View Answer play_arrow
question_answer 154) Chromosome was discovered by
A)
Hofmeister
done
clear
B)
Muller
done
clear
C)
Flemmmg
done
clear
D)
Hammeriing
done
clear
View Answer play_arrow
question_answer 155) Insectivorous plant is
A)
Cuscuta
done
clear
B)
Drosera
done
clear
C)
Striga
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 156) Mycoplasma is resistant for
A)
penicillin
done
clear
B)
tetracycline
done
clear
C)
streptomycin
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 157) The reduction division in chromosomes takes place in
A)
pollen grains
done
clear
B)
tapetum
done
clear
C)
megaspore cell
done
clear
D)
megaspore mother cell
done
clear
View Answer play_arrow
question_answer 158) Calvin cycle takes place in
A)
grana
done
clear
B)
stroma
done
clear
C)
intra thylakoid
done
clear
D)
matrix
done
clear
View Answer play_arrow
question_answer 159) The hormone, which prevents abscission is
A)
\[IBA\]
done
clear
B)
\[ABA\]
done
clear
C)
\[K-N\]
done
clear
D)
\[GA\]
done
clear
View Answer play_arrow
question_answer 160) Sagopalm is obtained from
A)
Pinus
done
clear
B)
Cycas
done
clear
C)
Ginkgo
done
clear
D)
Dateplam
done
clear
View Answer play_arrow
question_answer 161) Which of the following is not function of Golgi body?
A)
Protein synthesis
done
clear
B)
Formation of cell wall
done
clear
C)
Formation of fatty acids
done
clear
D)
Formation of plasma membrane
done
clear
View Answer play_arrow
question_answer 162) The RNA found in eukaryotic ribosomes are
A)
\[5S\]and\[16\,S\,RNA\]
done
clear
B)
\[5S,16\,S,\]\[18\,S\,RNA\]
done
clear
C)
\[2\,3S,16\,S\,RNA\]
done
clear
D)
\[5S\]and\[28\,S\,RNA\]
done
clear
View Answer play_arrow
question_answer 163) Water is not necessary for the fertilisation of
A)
algae
done
clear
B)
bryophyte
done
clear
C)
cycas
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 164) The biomass pyramid of forest ecosystem is
A)
linear
done
clear
B)
inverted
done
clear
C)
tetra angle
done
clear
D)
upright
done
clear
View Answer play_arrow
question_answer 165) After pollination the number of chromosome in egg will be
A)
\[n\]
done
clear
B)
\[2n\]
done
clear
C)
\[3n\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 166) Double fertilisation is the
A)
fertilisation of two eggs in an embryo sac
done
clear
B)
fusion of one male gamete with the egg cell and another male gamete with the secondary unclel
done
clear
C)
fusion of two male gametes with two polar nuclei
done
clear
D)
fusion of one male gamete with secondary nucleus
done
clear
View Answer play_arrow
question_answer 167) The significance of mitochondria was given
A)
Flemming
done
clear
B)
Moves
done
clear
C)
Mendel
done
clear
D)
Altman
done
clear
View Answer play_arrow
question_answer 168) Mitochondria contains
A)
only DNA
done
clear
B)
DNA and RNA
done
clear
C)
only RNA
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 169) Which of the following shows minimum number of chromosomes?
A)
\[n:1\]
done
clear
B)
\[n:2\]
done
clear
C)
\[n:3\]
done
clear
D)
\[n:4\]
done
clear
View Answer play_arrow
question_answer 170) Which of the following is formed in light reaction of photosynthesis?
A)
ATP
done
clear
B)
\[NADP{{H}_{2}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 171) In lysogenic phase which of the following takes place?
A)
Phage DNA integrated with host genome and does not multiplied
done
clear
B)
Phage DNA does not integrate with host genome
done
clear
C)
Phage DNA integrates with host genome and bacterial cell does not lyse
done
clear
D)
Phage DNA integrated with host DNA and multiplied
done
clear
View Answer play_arrow
question_answer 172) Who proposed fluid mosaic mode? of plasma membrane?
A)
Singer and Nicholson
done
clear
B)
Robertson
done
clear
C)
Benneson
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 173) The development of sporangium in Pteridium is
A)
a leptosporangiate type
done
clear
B)
an eusporangiate type
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 174) Respiratory roots (pneumatophores) are present in
A)
mesophytes
done
clear
B)
halophytes
done
clear
C)
xerophytes
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 175) The cell wall of fungi is formed by
A)
chitin and mannose
done
clear
B)
cellulose
done
clear
C)
glucose
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 176) Which is present in vascular bundle of Pmus?
A)
Tracheids
done
clear
B)
Vessels
done
clear
C)
Companion cells
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 177) Growth of stem in diameter is due to
A)
apical cell
done
clear
B)
lateral meristem
done
clear
C)
apical meristem
done
clear
D)
intercalary meristem
done
clear
View Answer play_arrow
question_answer 178) The plant group in which stomata opens in night is
A)
mesophytes
done
clear
B)
succulents
done
clear
C)
hydrophytes
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 179) The right equation for water potential is
A)
\[\psi ={{\psi }_{p}}-({{\psi }_{s}}+{{\psi }_{m}})\]
done
clear
B)
\[\psi ={{\psi }_{p}}+({{\psi }_{s}}-{{\psi }_{m}})\]
done
clear
C)
\[{{\psi }_{w}}={{\psi }_{s}}+{{\psi }_{p}}\]
done
clear
D)
\[{{\psi }_{w}}={{\psi }_{p}}+{{\psi }_{\pi }}+{{\psi }_{m}}\]
done
clear
View Answer play_arrow
question_answer 180) The respiratory quotient of fat is
A)
0.7
done
clear
B)
1.5
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow