question_answer 1) A step-up transformer has transformation ratio\[3:2\]. The voltage in the secondary coil, if the voltage in the primary is 30 V will be
A)
300V
done
clear
B)
90V
done
clear
C)
45V
done
clear
D)
23V
done
clear
View Answer play_arrow
question_answer 2)
In the inductive circuit given in the figure, the current rises after the switch is closed. At instant when the current is 15 mA, then potential difference across the inductor will be
A)
zero
done
clear
B)
240 V
done
clear
C)
180 V
done
clear
D)
60 V
done
clear
View Answer play_arrow
question_answer 3) A convex lens makes a real image of 4 cm long on a screen when the lens is shifted to a new position without disturbing the object or the screen, we get a real image on the screen, which is 16 cm long, then the length of the object is
A)
\[g/4\text{ }cm\]
done
clear
B)
8 cm
done
clear
C)
\[2g/3\]
done
clear
D)
2 cm
done
clear
View Answer play_arrow
question_answer 4) A man wants to slide down a rope. The breaking load for the rope is\[\frac{2}{3}\]rd of the weight of the man. With what minimum acceleration should fireman slide down?
A)
\[g/4\]
done
clear
B)
\[g/3\]
done
clear
C)
\[2g/3\]
done
clear
D)
\[g/6\]
done
clear
View Answer play_arrow
question_answer 5) A glass marble projected horizontally from the top of a table falls of a distance x from the edge of the table. If h is the height of the table, then the velocity of projection is
A)
\[h\sqrt{\frac{g}{2x}}\]
done
clear
B)
\[x\sqrt{\frac{g}{2h}}\]
done
clear
C)
\[gxh\]
done
clear
D)
\[gx+h\]
done
clear
View Answer play_arrow
question_answer 6) If\[x=\frac{{{\varepsilon }_{0}}lV}{t},\]where\[{{\varepsilon }_{0}}\]is the permittivity of free space,\[l\]is length, V is potential difference and \[t\]is time. The dimensions of\[x\]are same as that of
A)
charge
done
clear
B)
resistance
done
clear
C)
voltage
done
clear
D)
current
done
clear
View Answer play_arrow
question_answer 7) A body executes simple harmonic motion under the action of force\[{{F}_{1}}\]with a time period \[\frac{4}{5}\]s. If the force is changed to\[{{F}_{2}}\]it executes simple harmonic motion with time period\[\frac{3}{5}\]s. If both forces\[{{F}_{1}}\]and\[{{F}_{2}}\]act simultaneously in the same direction on the body, its time period will be
A)
\[\frac{12}{25}s\]
done
clear
B)
\[\frac{24}{25}s\]
done
clear
C)
\[\frac{35}{24}s\]
done
clear
D)
\[\frac{15}{12}s\]
done
clear
View Answer play_arrow
question_answer 8) A balloon contains\[500\text{ }{{m}^{3}}\]of He at\[27{}^\circ C\]and 1 atmospheric pressure. The volume of He at \[-3{}^\circ C\] and\[0.5\] atmospheric pressure will be
A)
\[700{{m}^{3}}\]
done
clear
B)
\[900{{m}^{3}}\]
done
clear
C)
\[1000\,{{m}^{3}}\]
done
clear
D)
\[500{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 9) If the radius of the earth is reduced by 1% without changing its mass. The change in g will be
A)
2% decrease
done
clear
B)
2% increases
done
clear
C)
1% decrease
done
clear
D)
1% increases
done
clear
View Answer play_arrow
question_answer 10) A Cannot engine works between temperature \[72{}^\circ C\]and\[27{}^\circ C\]. The efficiency of the engine is
A)
70%
done
clear
B)
30%
done
clear
C)
0%
done
clear
D)
10%
done
clear
View Answer play_arrow
question_answer 11) A man measure time period of a pendulum (T) in stationary lift. If the lift moves upward with acceleration\[g/4\], then new time period will be
A)
\[\sqrt{\frac{2}{5}}T\]
done
clear
B)
\[\sqrt{\frac{5}{2}}T\]
done
clear
C)
\[\frac{\sqrt{5}T}{2}\]
done
clear
D)
\[\frac{2T}{\sqrt{5}}\]
done
clear
View Answer play_arrow
question_answer 12) A wire of radius r has resistance R. If it is stretched to a radius of\[\frac{3r}{4}\]. Its resistance becomes
A)
\[\frac{256R}{81}\]
done
clear
B)
\[\frac{81R}{256}\]
done
clear
C)
\[\frac{16R}{9}\]
done
clear
D)
\[\frac{9R}{16}\]
done
clear
View Answer play_arrow
question_answer 13) An astronomical telescope has an objective of focal length 100 cm and magnifying power of the distance between the two lenses in normal adjustment will be
A)
106 cm
done
clear
B)
102 cm
done
clear
C)
92 cm
done
clear
D)
78 cm
done
clear
View Answer play_arrow
question_answer 14) What is the area of the plate of a 3 F parallel plate capacitor, if the separation between the plates is 5 mm?
A)
\[12.981\times {{10}^{2}}{{m}^{2}}\]
done
clear
B)
\[9.281\times {{10}^{9}}{{m}^{2}}\]
done
clear
C)
\[1.69\times {{10}^{12}}{{m}^{2}}\]
done
clear
D)
\[4.529\times {{10}^{9}}{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 15) A magnetizing field of 1600 A/m produces a magnetic; flux\[2.4\times {{10}^{-5}}\]Wb in an iron bar of cross-sectional area\[0.2\text{ }c{{m}^{2}}\]. The susceptibility of an iron bar will be
A)
1788
done
clear
B)
1192
done
clear
C)
596
done
clear
D)
298
done
clear
View Answer play_arrow
question_answer 16) If the earth field induction at a place is 0.36 gauss and the angle of dip is\[60{}^\circ ,\]the horizontal and vertical components of the field will be respectively
A)
0.18 gauss,\[0.18\sqrt{3}\]gauss
done
clear
B)
0.98 gauss,\[0.9\sqrt{2}\]gauss
done
clear
C)
1.08 gauss, and\[1.08\sqrt{3}\]gauss
done
clear
D)
1.07 gauss,\[0.11\sqrt{2}\]gauss
done
clear
View Answer play_arrow
question_answer 17) If a and b are two vectors, then the value of\[(a\times b)\times (a-b)\]is
A)
\[2(b\times a)\]
done
clear
B)
\[-2(b\times a)\]
done
clear
C)
\[b\times a\]
done
clear
D)
\[a\times b\]
done
clear
View Answer play_arrow
question_answer 18) A body of specific heat\[0.2\text{ }kcal/kg{}^\circ C\]is heated through\[100{}^\circ C\]. The percentage increase in its mass is
A)
9%
done
clear
B)
\[9.3\times {{10}^{-11}}%\]
done
clear
C)
10%
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 19) Which of the following is not correct regarding the radio telescope?
A)
It cannot work at night
done
clear
B)
It can detect a very faint radio signal
done
clear
C)
It can be operated even in cloudily weather
done
clear
D)
It is much cheaper than optical telescope
done
clear
View Answer play_arrow
question_answer 20) If the change in the value of g at a height h above the surface of the earth is the same as at a depth x below in surface, then
A)
\[x={{h}^{2}}\]
done
clear
B)
\[x=0.5h\]
done
clear
C)
\[x=2h\]
done
clear
D)
\[x=h\]
done
clear
View Answer play_arrow
question_answer 21) The sun delivers\[{{10}^{4}}W/{{m}^{2}}\]of electromagnetic flux to the earths surface. The total power that is incident on a roof of dimension \[(10\times 10){{m}^{2}}\]will be
A)
\[{{10}^{4}}W\]
done
clear
B)
\[{{10}^{5}}W\]
done
clear
C)
\[{{10}^{6}}W\]
done
clear
D)
\[{{10}^{7}}W\]
done
clear
View Answer play_arrow
question_answer 22) The minimum energy to ionize an atom is the energy required to
A)
add one electron to the atom
done
clear
B)
excite the atom from its ground state to its excited state
done
clear
C)
remove one outer most electron from the atom
done
clear
D)
remove one inner most electron from the atom
done
clear
View Answer play_arrow
question_answer 23) The distance between two point objects P and Q is 32 cm. A convex lens of focal length 15 cm is placed between them, so that the images of both the objects are formed at the same place. The distance of\[P\]from the lens could be
A)
20 cm
done
clear
B)
12 cm
done
clear
C)
18 cm
done
clear
D)
28 cm
done
clear
View Answer play_arrow
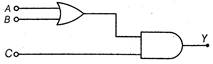
question_answer 24)
To get an output\[Y=1\]from circuit of figure, the input must be
A)
A-0 B-1 C-0
done
clear
B)
A-0 B-0 C-1
done
clear
C)
A-1 B-0 C-0
done
clear
D)
A-1 B-0 C-1
done
clear
View Answer play_arrow
question_answer 25) X-rays are not used for radar purposes because they are not
A)
reflected by target
done
clear
B)
partly absorbed by target
done
clear
C)
electromagnetic waves
done
clear
D)
completely absorbed by target
done
clear
View Answer play_arrow
question_answer 26) If\[E=at+b{{t}^{2}},\] what is the neutral temperature?
A)
\[-\frac{a}{2b}\]
done
clear
B)
\[+\frac{a}{2b}\]
done
clear
C)
\[-\frac{a}{b}\]
done
clear
D)
\[+\frac{a}{b}\]
done
clear
View Answer play_arrow
question_answer 27) An\[8\mu F\]capacitor is connected across 200 V, 50Hz line. What is the peak value of the charge through capacitor?
A)
\[2.5\times {{10}^{-3}}C\]
done
clear
B)
\[2.5\times {{10}^{-4}}C\]
done
clear
C)
\[5\times {{10}^{-5}}C\]
done
clear
D)
\[7.5\times {{10}^{-2}}C\]
done
clear
View Answer play_arrow
question_answer 28) Copper has face centered cubic (fcc) lattice with interatomic spacing equals to\[2.54\text{ }\overset{o}{\mathop{\text{A}}}\,\]. The value of lattice constant for this lattice is
A)
\[\text{3}\text{.59}\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[2.54\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1.27\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[5.08\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 29) In hydrogen atom, the electron in a given orbit has total energy\[-1.5eV\]. The potential energy is
A)
1.5eV
done
clear
B)
\[-1.5eV\]
done
clear
C)
3.0eV
done
clear
D)
\[-3.0eV\]
done
clear
View Answer play_arrow
question_answer 30) An oxide coated filament is useful in vacuum tubes because essentially
A)
it has high melting point
done
clear
B)
it can without and high temperature
done
clear
C)
it has good mechanical strength
done
clear
D)
it can emit electrons of relatively lower temperature
done
clear
View Answer play_arrow
question_answer 31) Dimension of\[\frac{L}{RCV}\]is
A)
\[[{{A}^{-1}}]\]
done
clear
B)
\[[{{A}^{-2}}]\]
done
clear
C)
\[[A]\]
done
clear
D)
\[[{{A}^{2}}]\]
done
clear
View Answer play_arrow
question_answer 32) The radii of curvature of the two surfaces of a lens are 20 cm and 30 cm and the reparative index of the material of the lens is 1.5. If the lens is concave-convex, then the focal length of the lens is
A)
24 cm
done
clear
B)
10 cm
done
clear
C)
15 cm
done
clear
D)
120 cm
done
clear
View Answer play_arrow
question_answer 33) A wire carrying current i and other carrying is in the same direction produce a magnetic field B at the midpoint. What will be the field when 2i current is switched off?
A)
B/2
done
clear
B)
2B
done
clear
C)
0
done
clear
D)
4B
done
clear
View Answer play_arrow
question_answer 34) The Reynolds number of a flow is the ratio of
A)
gravity to viscous force
done
clear
B)
gravity force to pressure force
done
clear
C)
inertia force to viscous force
done
clear
D)
viscous forces to pressure force
done
clear
View Answer play_arrow
question_answer 35) In a transistor circuit, the base current changes from\[30\mu A\]to\[90\mu A\]. If the current gain of the transistor is 30%, the change in the collector current is
A)
4 mA
done
clear
B)
2 mA
done
clear
C)
3.5 mA
done
clear
D)
1.8 mA
done
clear
View Answer play_arrow
question_answer 36) A drift velocity of free electrons in a conductor is\[{{\upsilon }_{d}}\], when the current i is flowing in it. If both the radius and current are doubled, the drift velocity will be
A)
\[\frac{{{v}_{d}}}{8}\]
done
clear
B)
\[\frac{{{v}_{d}}}{4}\]
done
clear
C)
\[\frac{{{v}_{d}}}{2}\]
done
clear
D)
\[{{v}_{d}}\]
done
clear
View Answer play_arrow
question_answer 37) The maximum range of a gun on horizontal terrain is 16 km if\[g=10\text{ }m/{{s}^{2}}\]. What must be the muzzle velocity of the shell?
A)
200 m/s
done
clear
B)
100 m/s
done
clear
C)
400 m/s
done
clear
D)
300 m/s
done
clear
View Answer play_arrow
question_answer 38) The length, breath and thickness of a block are given by\[l=12\text{ }cm,\text{ }b=6\text{ }cm\]and\[t=2.45\text{ }cm\]. The volume of the block according to the idea of significant figure should be
A)
\[1\times {{10}^{2}}c{{m}^{2}}\]
done
clear
B)
\[2\times {{10}^{2}}c{{m}^{3}}\]
done
clear
C)
\[1.763\times {{10}^{2}}c{{m}^{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 39) Two bulbs rated 200 W, 220V and 100 W, 220V, are connected in series, combination is connected to 220 V supply. Power consumed by the circuit is
A)
80 W
done
clear
B)
67 W
done
clear
C)
76 W
done
clear
D)
65 W
done
clear
View Answer play_arrow
question_answer 40) In a full wave rectifier, input AC current has a frequency v. The output frequency of current is
A)
\[\frac{v}{2}\]
done
clear
B)
\[v\]
done
clear
C)
\[2v\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 41) When the distance between slits of Youngs double slit experiment is reduced to half then the fringe middle will become
A)
constant
done
clear
B)
four times
done
clear
C)
two times
done
clear
D)
half
done
clear
View Answer play_arrow
question_answer 42) Magnetic field intensify at the centre of coil of 50 turns, 0.5m radius and carrying a current of 2A, is
A)
\[5\times {{10}^{-5}}T\]
done
clear
B)
\[3\times {{10}^{-5}}T\]
done
clear
C)
\[1.25\times {{10}^{-4}}T\]
done
clear
D)
\[0.5\times {{10}^{-5}}T\]
done
clear
View Answer play_arrow
question_answer 43) The minimum wavelength of X-ray emitted by X-ray tube is\[0.4125\text{ }\overset{o}{\mathop{\text{A}}}\,\]. The accelerating voltage is
A)
30kV
done
clear
B)
50kV
done
clear
C)
80RV
done
clear
D)
60kV
done
clear
View Answer play_arrow
question_answer 44) A monoatomic gas supplied the heat Q very slowly keeping the pressure constant. The work done by the gas will be
A)
\[\frac{2}{3}Q\]
done
clear
B)
\[\frac{3}{5}Q\]
done
clear
C)
\[\frac{2}{5}Q\]
done
clear
D)
\[\frac{1}{5}Q\]
done
clear
View Answer play_arrow
question_answer 45) Light of wavelength 488 nm is produced by organ laser, which is used in the photoelectric effect. When light from this spectral line is incident on the emitter, the stopping (cut-off) potential of photoelectron is 0.38 V. Find the work function of the material from which the emitter is made.
A)
1.25eV
done
clear
B)
2.17eV
done
clear
C)
4.07eV
done
clear
D)
3.57eV
done
clear
View Answer play_arrow
question_answer 46)
A mass m is suspended from the two springs of spring constants\[{{k}_{1}}\]and\[{{k}_{2}}\]as shown. The time period of vertical oscillations of the mass will be
A)
\[2\pi \sqrt{\left( \frac{{{k}_{1}}+{{k}_{2}}}{m} \right)}\]
done
clear
B)
\[2\pi \sqrt{\left( \frac{m}{{{k}_{1}}+{{k}_{2}}} \right)}\]
done
clear
C)
\[2\pi \sqrt{\frac{m({{k}_{1}}{{k}_{2}})}{{{k}_{1}}+{{k}_{2}}}}\]
done
clear
D)
\[2\pi \sqrt{\frac{m({{k}_{1}}+{{k}_{2}})}{({{k}_{1}}{{k}_{2}})}}\]
done
clear
View Answer play_arrow
question_answer 47) A copper wire and a steel wire of the same diameter and length are connected end to end and a force is applied which stretches their combined length by 1 cm. Then the two wires will have
A)
the same stress and strain
done
clear
B)
the same stress but different strain
done
clear
C)
the same strain but difference stress
done
clear
D)
difference, stress and strain
done
clear
View Answer play_arrow
question_answer 48) Which of the following have higher specific change?
A)
Positorn
done
clear
B)
Proton
done
clear
C)
He
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 49) A beam of light after reflection on a plane mirror forms a real image. The beam is
A)
convergent
done
clear
B)
divergent
done
clear
C)
parallel to axis
done
clear
D)
parallel but not to axis of mirror
done
clear
View Answer play_arrow
question_answer 50) A straight conductor of length 0.4 m is moved with a speed of 7 m/s perpendicular to the magnetic field of intensity of\[0.9\text{ }Wb/{{m}^{2}}\]. The induced emf across the conductor will be
A)
7.25V
done
clear
B)
5.54V
done
clear
C)
1,25V
done
clear
D)
2.52V
done
clear
View Answer play_arrow
question_answer 51) If the heat of 110 J is added to a gaseous system, whose internal energy is 40 J. Then the amount of external work done is
A)
140J
done
clear
B)
70J
done
clear
C)
110J
done
clear
D)
150J
done
clear
View Answer play_arrow
question_answer 52) The nature of light waves is similar to
A)
cosmic rays
done
clear
B)
cathode rays
done
clear
C)
gamma rays
done
clear
D)
alpha rays
done
clear
View Answer play_arrow
question_answer 53) A body is thrown with a velocity of 9.8 m/s making an angle of \[30{}^\circ \] with the horizontal. It will hit the ground after a time
A)
1.5s
done
clear
B)
1s
done
clear
C)
3s
done
clear
D)
2s
done
clear
View Answer play_arrow
question_answer 54) Rain drops are spherical in shape due to
A)
acceleration due to gravity
done
clear
B)
downward motion
done
clear
C)
surface tension
done
clear
D)
capillary
done
clear
View Answer play_arrow
question_answer 55) For molecules of a gas have speeds 1, 2, 3 and 4 km/s. The value of the root mean square speed of the gas molecules is
A)
\[\frac{1}{2}\sqrt{15}\]km/s
done
clear
B)
\[\frac{1}{2}\sqrt{10}\]km/s
done
clear
C)
2.5km/s
done
clear
D)
\[\frac{\sqrt{15}}{2}\]km/s
done
clear
View Answer play_arrow
question_answer 56) If\[\sigma =\]surface change density,\[\varepsilon =\]electric permittivity, the dimensions of\[\frac{\sigma }{\varepsilon }\]is same as
A)
electric force
done
clear
B)
electric field intensity
done
clear
C)
pressure
done
clear
D)
electric charge
done
clear
View Answer play_arrow
question_answer 57) A conducting sphere of radius 30 cm is given a charge of\[1.2\times {{10}^{-8}}C\]. What will be its potential?
A)
0.03kV
done
clear
B)
0.9kV
done
clear
C)
1.8kV
done
clear
D)
3.6kV
done
clear
View Answer play_arrow
question_answer 58) A simple pendulum of length\[l\]has a maximum angular displacement Q. The maximum kinetic energy of the bob is
A)
\[mgf(1-cos\theta )\]
done
clear
B)
\[0.5\text{ }mgl\]
done
clear
C)
\[mgl\]
done
clear
D)
\[2\,mgl\]
done
clear
View Answer play_arrow
question_answer 59) Radius of orbit of satellite of earth is R its kinetic energy is proportional to
A)
\[\frac{1}{R}\]
done
clear
B)
\[\frac{1}{\sqrt{R}}\]
done
clear
C)
\[R\]
done
clear
D)
\[\frac{1}{{{R}^{3}}}\]
done
clear
View Answer play_arrow
question_answer 60) The energy of an X-ray photon is 2 keV, then its frequency will be
A)
\[3.2\times {{10}^{-6}}\]per second
done
clear
B)
\[5\times {{10}^{17}}\]per second
done
clear
C)
\[2\times {{10}^{17}}\]per second
done
clear
D)
\[2\times {{10}^{18}}\]8 per second
done
clear
View Answer play_arrow
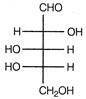
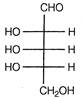
question_answer 61) Which of the following statements is not an essential feature of an optically active molecule?
A)
It will rotate the plane of polarised light
done
clear
B)
It will have a non-superimposable mirror image
done
clear
C)
It will have no element of symmetry
done
clear
D)
It will have an asymmetric carbon atom
done
clear
View Answer play_arrow
question_answer 62) The\[p{{K}_{a}}\]of phenol is 10. Which of the following bases will deprotonate phenol Completely? \[NaN{{H}_{2}}(p{{K}_{a}}ofN{{H}_{3}}=35),NaOH(p{{K}_{a}}\]of\[{{H}_{2}}O=16),\]\[Na{{H}_{2}}(p{{K}_{a}}ofNH_{4}^{+}=10),\]\[NaOOCC{{H}_{3}}\] \[(p{{K}_{a}}ofC{{H}_{3}}COOH=5)\]
A)
\[NaN{{H}_{2}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[NaN{{H}_{2}}\]and\[NaOH\]
done
clear
D)
\[N{{H}_{3}}\]and \[NaOCOC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 63) Which one of the following is the most stable reactive intermediate?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 64) The reagent which could distinguish between 1-hexyne and 1-hexene is
A)
\[Ag(N{{H}_{3}})_{2}^{+}\]
done
clear
B)
\[KMn{{O}_{4}}\]
done
clear
C)
\[B{{r}_{2}}\]in\[CC{{l}_{4}}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
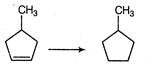
question_answer 65)
Choose the correct reagent required to carry out the transformation.
A)
Zn + cone.\[HCl\]
done
clear
B)
Cone. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
Li then\[{{H}_{2}}O\]
done
clear
D)
\[{{H}_{2}}/Pt\]
done
clear
View Answer play_arrow
question_answer 66) \[N{{O}_{2}}\] (brown colour gas) exists in equilibrium with\[{{N}_{2}}{{O}_{4}}\](colourless gas) as gjlven by chemical equation. \[\underset{(brown)}{\mathop{2N{{O}_{2}}}}\,\underset{(colourless)}{\mathop{{{N}_{2}}{{O}_{4}}}}\,\] Mixture is slightly brown due to existence of\[N{{O}_{2}}\]. If pressure is increased
A)
colour intensity is increased
done
clear
B)
colour intensity is decreased
done
clear
C)
colour intensity first increases and then decreases
done
clear
D)
No change in colour intensity
done
clear
View Answer play_arrow
question_answer 67) Spontaneous adsorption of gas on solid surface is an exothermic process because
A)
\[\Delta H\]increases for system
done
clear
B)
\[\Delta S\]increases for gas
done
clear
C)
\[\Delta S\]decreases for gas
done
clear
D)
\[\Delta G\]increases for gas
done
clear
View Answer play_arrow
question_answer 68) \[\Delta H_{f}^{o}({{C}_{2}}{{H}_{5}}),\Delta H_{f}^{o}({{C}_{2}}{{H}_{6}})\]are\[{{x}_{1}}\]and\[{{x}_{2}}\]kcal \[mo{{l}^{-1}}\]respectively. Then heat of hydrogenation of \[{{C}_{2}}{{H}_{4}}\]is
A)
\[{{x}_{1}}+{{x}_{2}}\]
done
clear
B)
\[{{x}_{1}}-{{x}_{2}}\]
done
clear
C)
\[-({{x}_{1}}-{{x}_{2}})\]
done
clear
D)
\[{{x}_{1}}+2{{x}_{2}}\]
done
clear
View Answer play_arrow
question_answer 69) The heat of formation of\[{{C}_{12}}{{H}_{22}}{{O}_{11}}(s),C{{O}_{2}}(g)\] and\[{{H}_{2}}O(l)\]are\[-530,-94.3\]and\[-68.3\]kcal \[mo{{l}^{-1}}\]respectively. The amount of\[{{C}_{12}}{{H}_{22}}{{O}_{11}}\]to supply 2700 kcal of energy is
A)
382.70 g
done
clear
B)
832.74 g
done
clear
C)
463.9 g
done
clear
D)
684.0 g
done
clear
View Answer play_arrow
question_answer 70) 0.1 M solution of\[C{{H}_{3}}COOH\]should be diluted to how many times so that pH is doubled?
A)
4.0 times
done
clear
B)
\[5.55\times {{10}^{4}}\]times
done
clear
C)
\[5.55\times {{10}^{6}}\]times
done
clear
D)
\[{{10}^{-2}}\]times
done
clear
View Answer play_arrow
question_answer 71) If the equilibrium constant of the reaction of weak acid HA with strong base is\[{{10}^{9}},\]then pH of 0.1 M NaA is
A)
5
done
clear
B)
9
done
clear
C)
7
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 72) Which is maximum hydrated?
A)
\[NaCl\]
done
clear
B)
\[MgC{{l}_{2}}\]
done
clear
C)
\[AlC{{l}_{3}}\]
done
clear
D)
\[SiC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 73) For the process,\[X(g)+{{e}^{-}}\xrightarrow[{}]{{}}{{X}^{-}}(g);\Delta H=x\]and \[{{X}^{-}}(g)\xrightarrow[{}]{{}}X(g)+{{e}^{-}};\Delta H=y\]select correct alternate.
A)
lonisation energy of\[{{X}^{-}}(g)\] is y
done
clear
B)
Electron affinity of\[X(g)\]is\[x\]
done
clear
C)
Electron affinity of\[X(g)\]is\[-y\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 74) Mass of one atom is\[6.66\times {{10}^{-23}}g\]. Its percentage in an hydride is 95.24. Thus, hydride is
A)
MH
done
clear
B)
\[M{{H}_{2}}\]
done
clear
C)
\[M{{H}_{3}}\]
done
clear
D)
\[M{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 75) Which can dissolve\[{{I}_{2}}\]?
A)
\[KI\]
done
clear
B)
\[NaI\]
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 76) Ozone layer is being depleted. This is due to
A)
No (nitric oxide) emission from supersonic jets
done
clear
B)
chlorofluoro carbon used as aerosols
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 77) The oxygen carrying pigment, oxy-haemocyanin containing two copper ions is diamagnetic, because
A)
the two copper ions are in + 1 oxidation state
done
clear
B)
one copper ion is in + 1 oxidation state while other is in + 2 oxidation state
done
clear
C)
of the strong antiferromagnetic interactions between two copper ions
done
clear
D)
of the ferromagnetic interactions between the two copper ions
done
clear
View Answer play_arrow
question_answer 78) Which is not true statement?
A)
Ions of d-block elements are coloured due to d-d transition
done
clear
B)
lons of\[f-\]block elements are coloured due to\[f-f\]transition
done
clear
C)
\[{{[Sc{{({{H}_{2}}O)}_{6}}]}^{3+}},{{[Ti{{({{H}_{2}}O)}_{6}}]}^{4+}}\]are coloured complexes
done
clear
D)
\[C{{u}^{+}}\] is colourless ion
done
clear
View Answer play_arrow
question_answer 79) The crystal field splitting for\[C{{r}^{3+}}\]ion in an octahedral field increases for ligands \[{{I}^{-}},{{H}_{2}}O,\] \[N{{H}_{3}},C{{N}^{-}}\] and the order is
A)
\[{{\text{I}}^{-}}<{{H}_{2}}O<N{{H}_{3}}<C{{N}^{-}}\]
done
clear
B)
\[C{{N}^{-}}<{{\text{I}}^{-}}<{{H}_{2}}O<N{{H}_{3}}\]
done
clear
C)
\[C{{N}^{-}}<N{{H}_{3}}<{{H}_{2}}O<{{I}^{-}}\]
done
clear
D)
\[N{{H}_{3}}<{{H}_{2}}O<{{I}^{-}}<C{{N}^{-}}\]
done
clear
View Answer play_arrow
question_answer 80) The reaction between metallic silver and aqueous\[NaCN\]forming a soluble complex occurs in the presence of
A)
nitrogen
done
clear
B)
oxygen
done
clear
C)
helium
done
clear
D)
chlorine
done
clear
View Answer play_arrow
question_answer 81) A 1 L flask contains 32 g\[{{O}_{2}}\]gas at\[27{}^\circ C\]. What mass of\[{{O}_{2}}\]must be released to reduce the pressure in the flask to 12.315 atm?
A)
8 g
done
clear
B)
16 g
done
clear
C)
24 g
done
clear
D)
32 g
done
clear
View Answer play_arrow
question_answer 82) 1 mol of\[{{O}_{2}}\]and\[x\]mol of Ne in a 10 L flask at constant temperature exert a pressure of 10 atm. If partial pressure of\[{{O}_{2}}\]is 2 atm, moles of Ne in the mixture is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 83) A 25 W bulb emits monochromatic yellow light of wavelength of 0.57pm. Calculate the rate of emission of quanta per second.
A)
\[7.169\times {{10}^{19}}\]
done
clear
B)
\[4.569\times {{10}^{19}}\]
done
clear
C)
\[8.579\times {{10}^{19}}\]
done
clear
D)
\[9.662\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 84) The correct set of quantum numbers for the unpaired electron of a chlorine atom is
A)
\[2,0,0,+\frac{1}{2}\]
done
clear
B)
\[2,1,-1,+\frac{1}{2}\]
done
clear
C)
\[3,1,-1,\pm \frac{1}{2}\]
done
clear
D)
\[3,0,0,\pm \frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 85) Calculate the radius of a water molecule assuming to be spherical (density\[=1\text{ }g\text{ }m{{L}^{-1}}\]).
A)
\[1.925\times {{10}^{-8}}cm\]
done
clear
B)
\[1.925\times {{10}^{-9}}cm\]
done
clear
C)
\[1.925\times {{10}^{-10}}cm\]
done
clear
D)
\[1.925\times {{10}^{-11}}cm\]
done
clear
View Answer play_arrow
question_answer 86) When 6g of a monobasic acid is dissolved in 100 mL solution, it is 1 N. What is equivalent mass of the acid?
A)
\[20\text{ }gequi{{v}^{-1}}\]
done
clear
B)
\[40\text{ }gequi{{v}^{-1}}\]
done
clear
C)
\[60\text{ }gequi{{v}^{-1}}\]
done
clear
D)
\[80\text{ }gequi{{v}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 87) Each\[N{{H}_{3}}\]molecule has six other\[N{{H}_{3}}\]molecules as nearest neighbours in solid state; AH of sublimation of \[N{{H}_{3}}\] molecule at the m. p is\[30.8\text{ }kJ\text{ }mo{{l}^{-1}}\]and in the absence of H-bonding estimated\[\Delta H\]of sublimation is\[14.4\text{ }kJ\text{ }mo{{l}^{-1}}\]. Hence, strength of H-bond in solid\[N{{H}_{3}}\]is
A)
\[5.47\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
B)
\[\text{10}\text{.93 }kJ\text{ }mo{{l}^{-1}}\]
done
clear
C)
\[\text{16}\text{.40 }kJ\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[\text{-16}\text{.4 }kJ\text{ }mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 88) A mineral having the formula\[A{{B}_{2}}\]crystallizes in the cubic close-packed lattice, with the A atoms occupying the lattice points. The fraction of the tetrahedral sites occupied by B atoms is
A)
20%
done
clear
B)
40%
done
clear
C)
60%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 89) Which of the following is true regarding teflon?
A)
It is a linear, unbranched polymer of tetrafluoro ethylene
done
clear
B)
It has very high thermal stability
done
clear
C)
Polymer molecules are associated by strong dipole-dipole attraction
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 90) Cellulose has very high degree of hydrophilicity because of
A)
its amorphous nature
done
clear
B)
crystalline nature
done
clear
C)
presence of excessive voids in solid state
done
clear
D)
presence of many hydroxyl groups on the polymer back bone
done
clear
View Answer play_arrow
question_answer 91) By adding sodium dodecyl sulphate, during the electrophoresis of proteins, it is possible to
A)
determine a proteins isoelectric point
done
clear
B)
determine an enzymes specific activity
done
clear
C)
determine the amino acid composition of the protein
done
clear
D)
presence a proteins native structure and biological activity
done
clear
View Answer play_arrow
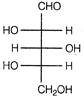
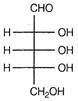
question_answer 92) Which of the following aldoses gives an optically active compound upon reaction with warm dilute nitric acid?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 93) In the a-helix, the hydrogen bonds
A)
are roughly parallel to the axis of the helix
done
clear
B)
are roughly perpendicular to the axis of the helix
done
clear
C)
occur mainly between electronegative atoms of the R groups
done
clear
D)
occur only between some of the amino acids of the helix
done
clear
View Answer play_arrow
question_answer 94) Which one of the following antioxidant commonly used to increase the storage life of butter?
A)
Butylated hydroxy anisol
done
clear
B)
Sodium sulphite
done
clear
C)
Sodium metabisulphite
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 95) Which of the following compounds is the weakest Bronsted base?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 96) \[C{{H}_{3}}CHO\xrightarrow[{}]{oxidatton}(A)\xrightarrow[{}]{PC{{l}_{5}}}(B)\xrightarrow[{}]{C{{H}_{3}}COONa}\] Compound is
A)
\[{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
B)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}COOC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 97) The strongest acid among the following compound is
A)
\[HCOOH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CH(Cl)COOH\]
done
clear
C)
\[ClC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
D)
\[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 98) Phenol, when it first react with cone. Sulphuric acid and then with cone. nitric acid gives
A)
nitrobenzene
done
clear
B)
2,4,6-trinitrobenzene
done
clear
C)
o-nitrophenol
done
clear
D)
p-nitrophenol
done
clear
View Answer play_arrow
question_answer 99) During dehydration of alcohols to alkenes by heating with cone. 112804, the initial step is
A)
formation of an ester
done
clear
B)
protonation of alcohol molecule
done
clear
C)
formation of carbocation
done
clear
D)
elimination of water
done
clear
View Answer play_arrow
question_answer 100)
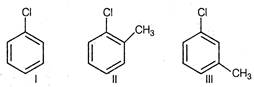
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
A)
\[I<II<III\]
done
clear
B)
\[I<III<II\]
done
clear
C)
\[III<II<I\]
done
clear
D)
\[II<III<I\]
done
clear
View Answer play_arrow
question_answer 101) Which one of the following is incorrect?
A)
lonisation enthalpy of molecular oxygen is very close to that of\[Xe\]
done
clear
B)
Only type of interatomic interaction between particles of noble gases are weak dispersion forces
done
clear
C)
Hydrolysis of \[Xe{{F}_{6}}\] is not a redox reaction
done
clear
D)
Xenon fluorides are not reactive
done
clear
View Answer play_arrow
question_answer 102) Which one of the following is not tetrahedral in shape?
A)
\[SiC{{l}_{4}}\]
done
clear
B)
\[NH_{4}^{+}\]
done
clear
C)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
D)
\[S{{F}_{4}}\]
done
clear
View Answer play_arrow
question_answer 103) Which one of the following nitrogen oxides does not contain\[N-N\]bonds?
A)
\[{{N}_{2}}O\]
done
clear
B)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
D)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 104) Extent of adsorption of adsorbate from solution phase increases with
A)
increase in amount of adsorbate in solution
done
clear
B)
decrease in surface area of adsorbent
done
clear
C)
increase in temperature of solution
done
clear
D)
decrease in amount of adsorbate in solution
done
clear
View Answer play_arrow
question_answer 105) Which of the following is a negatively charged sol?
A)
Sol of charcoal
done
clear
B)
Haemoglobin
done
clear
C)
\[Ti{{O}_{2}}\] sol
done
clear
D)
Basic dye stuffs
done
clear
View Answer play_arrow
question_answer 106) Which of the following elements is present as the impurity to the maximum extent in w iron?
A)
Phosphorus
done
clear
B)
Manganese
done
clear
C)
Carbon
done
clear
D)
Silicon
done
clear
View Answer play_arrow
question_answer 107) Which metallurgy does not involve leaching?
A)
Fe
done
clear
B)
Ag
done
clear
C)
Au
done
clear
D)
\[Al\]
done
clear
View Answer play_arrow
question_answer 108) The role of a catalyst is to change
A)
enthalpy of reaction
done
clear
B)
equilibrium constant
done
clear
C)
activation energy of reaction
done
clear
D)
Gibbs energy of reaction
done
clear
View Answer play_arrow
question_answer 109) Which one of the following options is not correct for the decomposition reaction of\[N{{H}_{3}}\]? \[2N{{H}_{3}}(g)\xrightarrow[Pt\text{ }catalyst]{1130K,at\,high\,p}{{N}_{2}}(g)+3{{H}_{2}}(g)\]
A)
Rate of reaction = rate constant
done
clear
B)
Further increase in pressure will change the rate of reaction
done
clear
C)
Rate of decomposition of\[N{{H}_{3}}\]will remain constant until \[N{{H}_{3}}\]disappears completely
done
clear
D)
Rate of reaction depends on concentration of\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 110) For the reaction,\[a+A+bB\xrightarrow[{}]{{}}cC,\]if \[-3\frac{d[A]}{dt}\] \[=-3\frac{d[B]}{dt}=+1.5\frac{d[C]}{dt}\]then a, b and c respectively are
A)
1, 3, 2
done
clear
B)
1, 2, 3
done
clear
C)
3, 2, 1
done
clear
D)
3, 1, 2
done
clear
View Answer play_arrow
question_answer 111) When 2.495 g of\[CuS{{O}_{4}}.x{{H}_{2}}O\](molar mass 249.5) is heated, 0.05 mole of\[{{H}_{2}}O\]is lost. Therefore,\[x\]is
A)
one
done
clear
B)
three
done
clear
C)
five
done
clear
D)
seven
done
clear
View Answer play_arrow
question_answer 112) \[{{N}_{p}}\]is isoelectroriic to\[C{{O}_{2}}\]and\[N_{3}^{-}\]. Which of the following is the structure of\[{{N}_{2}}O\]?
A)
done
clear
B)
\[N-O-N\]
done
clear
C)
\[N-N-O\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 113) Number of lone pair in\[XeO{{F}_{4}}\]is
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
three
done
clear
View Answer play_arrow
question_answer 114) Which of the following is a favourable factor for cation formation?
A)
Low ionisation potential
done
clear
B)
High electron affinity
done
clear
C)
High electronegativity
done
clear
D)
Small atomic size
done
clear
View Answer play_arrow
question_answer 115) 60 g of urea is dissolved in 1100 g solution. To keep\[\Delta {{T}_{f}}/{{K}_{f}}\]as 1 mol\[k{{g}^{-1}}\], water separated in the form of ice is
A)
40 g
done
clear
B)
60 g
done
clear
C)
100 g
done
clear
D)
200 g
done
clear
View Answer play_arrow
question_answer 116) Osmosis involved in
A)
excretion of urine
done
clear
B)
interchange of nutrients and waste products betweeen tissue cells and their surroundings
done
clear
C)
in both cases
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 117) Vapour density of\[PC{{l}_{5}}(g)\]dissociating into \[PC{{l}_{3}}(g)\]and\[C{{l}_{2}}(g)\]is 100. Hence, vant Hoff factor for the case \[PC{{l}_{5}}(g)PC{{l}_{3}}(g)+C{{l}_{2}}(g)\] is
A)
1.85
done
clear
B)
3.70
done
clear
C)
1.085
done
clear
D)
1.0425
done
clear
View Answer play_arrow
question_answer 118) Ionic conductances of \[{{H}^{+}}\] and\[SO_{4}^{2-}\]at infinite dilution are\[x\]and y\[S\,c{{m}^{2}}equi{{v}^{-1}}\]. Hence, equivalent conductance of\[{{H}_{2}}S{{O}_{4}}\]at infinite dilution is
A)
\[(x+y)\]
done
clear
B)
\[2(x+y)\]
done
clear
C)
\[2x+y\]
done
clear
D)
\[x+2y\]
done
clear
View Answer play_arrow
question_answer 119) In the refining of silver by electrolytic method, what will be the weight of 100 g Ag anode if 5A current is passed for hours? The purity of silver anode is 95% by weight.
A)
42.91 g
done
clear
B)
57.59 g
done
clear
C)
13.69g
done
clear
D)
36.38 g
done
clear
View Answer play_arrow
question_answer 120) Salt bridge contains
A)
calomel
done
clear
B)
corrosive sublimate
done
clear
C)
agar-agar paste
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 121) A function of lysosomes is
A)
replication
done
clear
B)
hydrolysis
done
clear
C)
respiration
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 122) Mitochondria and chloroplasts are similar because
A)
both have 80S ribosomes
done
clear
B)
both have nuclei
done
clear
C)
both have single membrane envelope
done
clear
D)
both have DNA
done
clear
View Answer play_arrow
question_answer 123) The oldest fossil belongs to which of the following group of plants/animals?
A)
Asteriodae
done
clear
B)
Rhodophyceae
done
clear
C)
Blue green algae
done
clear
D)
Phaeophyceae
done
clear
View Answer play_arrow
question_answer 124) The term phylum was given by
A)
Haeckel
done
clear
B)
Linnaeus
done
clear
C)
Cuvier
done
clear
D)
Theophrastus
done
clear
View Answer play_arrow
question_answer 125) Mehlis gland in tapeworm is associated with
A)
respiration
done
clear
B)
reproduction
done
clear
C)
excretion
done
clear
D)
circulation
done
clear
View Answer play_arrow
question_answer 126) Which of the following animals has a tetramorphic colony?
A)
Velella
done
clear
B)
Physalia
done
clear
C)
Obelia
done
clear
D)
Porpita
done
clear
View Answer play_arrow
question_answer 127) Viral genome incorporated into host DNA is called
A)
prophase
done
clear
B)
plasmid
done
clear
C)
prophage
done
clear
D)
bacteriophage
done
clear
View Answer play_arrow
question_answer 128) Select the correct set of animals of class-Mammalia.
A)
Lion, bat. whale, ostrich
done
clear
B)
Whale, bat, kangaroo, hippopotamus
done
clear
C)
Lion, hippopotamus, penguin, bat
done
clear
D)
Hippopotamus, penguin, whale, kangaroo
done
clear
View Answer play_arrow
question_answer 129) Which of the following statements regarding universal rules of nomenclature is wrong?
A)
The first word denoting the genus starts with a capital letter
done
clear
B)
The first word in a biological name represents the genus
done
clear
C)
Biological names are generally in Greek and written in italics
done
clear
D)
Both the words in a biological name, when hand-written, are separately underlined
done
clear
View Answer play_arrow
question_answer 130) Select the type of enzyme involved in the following reaction. \[S-G+S\xrightarrow[{}]{{}}S+S-G\]
A)
Lyase
done
clear
B)
Transferase
done
clear
C)
Hydrolase
done
clear
D)
Dehydrogenase
done
clear
View Answer play_arrow
question_answer 131) Ringers solution contains
A)
\[N{{a}^{+}}+C{{l}^{-}}\] ions
done
clear
B)
\[N{{a}^{+}}+{{K}^{+}}\] ions
done
clear
C)
\[N{{a}^{+}}+{{K}^{+}}+C{{l}^{-}}\]ions
done
clear
D)
\[{{K}^{+}}+C{{l}^{-}}\]ions
done
clear
View Answer play_arrow
question_answer 132)
Match the column I with column II and choose the correct option. Column I Column II A. Goblet cells 1. Antibacterial agent B. Lysozyme 2. Mucous C. Saliva 3. \[HCl\] D. Oxyntic cells 4. Sublingual gland
Codes
A)
A-3 B-1 C-4 D-2
done
clear
B)
A-2 B-3 C-1 D-4
done
clear
C)
A-1 B-3 C-4 D-2
done
clear
D)
A-2 B-1 C-4 D-3
done
clear
View Answer play_arrow
question_answer 133) According to Boyles law, the product of pressure and volume is a constant. Hence,
A)
if volume of lungs is increased, the pressure decreases disproportionately
done
clear
B)
if volume of lungs is increased, the pressure remains the same
done
clear
C)
if volume of lungs is increased, the pressure decreases proportionately
done
clear
D)
if volume of lungs is increased, the pressure also increases proportionately
done
clear
View Answer play_arrow
question_answer 134) Ivan Pavlov performed experiments on
A)
origin of life
done
clear
B)
cardiac reflexes
done
clear
C)
simple reflexes
done
clear
D)
conditioned reflexes
done
clear
View Answer play_arrow
question_answer 135) Marasmus in children is caused by deficiency of
A)
protein
done
clear
B)
fats
done
clear
C)
vitamins
done
clear
D)
carbohydrates
done
clear
View Answer play_arrow
question_answer 136)
Given below are four methods (A to D) and their modes of action (1 to 4) in achieving contraception. Select their correct matching from the four options that follow. Column I Column II A. The Pill 1. Prevents sperms reaching cervix B. Condom 2. Prevents implantation C. Vasectomy 3. Prevents ovulation D. Copper-T 4. Semen contains no sperms
Codes
A)
A-3, B-4, C-1, D-2
done
clear
B)
A-2, B-3, C-1, D-4
done
clear
C)
A-3, B-1, C-4, D-2
done
clear
D)
A-4,B-1,C-2,D-3
done
clear
View Answer play_arrow
question_answer 137) Bilirubin and biliverdin are
A)
enzymes
done
clear
B)
bile salts
done
clear
C)
bile pigments
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 138) When the organic acid is oxidised in respiration, the value of R.Q. becomes
A)
zero
done
clear
B)
one
done
clear
C)
less than one
done
clear
D)
more than one
done
clear
View Answer play_arrow
question_answer 139) The crossing of a homozygous tall pea plant and homozygous dwarf pea plant would yield plants in the ratio of
A)
2 tall: 2 dwarf
done
clear
B)
all heterozygous tall
done
clear
C)
all homozygous dwarf
done
clear
D)
one homozygous tall; one homozygous dwarf; two heterozygous tall
done
clear
View Answer play_arrow
question_answer 140) The off springs obtained by mating two pure strains having constrasting characters are called as
A)
mutants
done
clear
B)
hybrids
done
clear
C)
\[{{F}_{2}}-\]generation
done
clear
D)
P-generation
done
clear
View Answer play_arrow
question_answer 141) Which of the following genotypes does not produce a sugar polymer on the surface of the RBCs?
A)
\[{{I}^{A}}{{I}^{A}}\]
done
clear
B)
\[{{I}^{A}}{{I}^{i}}\]
done
clear
C)
\[ii\]
done
clear
D)
\[{{I}^{A}}{{I}^{B}}\]
done
clear
View Answer play_arrow
question_answer 142) Tectonic is the study of
A)
volcanoes
done
clear
B)
sand dunes
done
clear
C)
earthquakes
done
clear
D)
earths crust
done
clear
View Answer play_arrow
question_answer 143) Recognition sequence in Eco RI is
A)
GGCC
done
clear
B)
GAATTC
done
clear
C)
AAGCTT
done
clear
D)
CTGCAG
done
clear
View Answer play_arrow
question_answer 144) During gene cloning, which is called gene taxi?
A)
Plasmid
done
clear
B)
Protozoa
done
clear
C)
Vaccine
done
clear
D)
Bacterium
done
clear
View Answer play_arrow
question_answer 145) Flavr savr variety of tomato is a
A)
mutated form
done
clear
B)
somaclonal variety
done
clear
C)
transgenic crop
done
clear
D)
high yielding variety
done
clear
View Answer play_arrow
question_answer 146) If the decomposers become extinct, the most severally affected would be
A)
bio magnification
done
clear
B)
damage to nitrogen fixation
done
clear
C)
non-cycling of minerals
done
clear
D)
carnivores will be starved
done
clear
View Answer play_arrow
question_answer 147) Which of the following is false?
A)
The energy content in a trophic level is determined by considering individuals of a species in that trophic level
done
clear
B)
The succession that occur in newly cooled lava is called primary success
done
clear
C)
Rate of succession is faster in secondary succession
done
clear
D)
Quantity of biomass in a trophic level at a particular period is called as stop-(standing) crop
done
clear
View Answer play_arrow
question_answer 148) Ratio between mortality and natality is called
A)
population ratio
done
clear
B)
vital index
done
clear
C)
census ratio
done
clear
D)
density coefficient
done
clear
View Answer play_arrow
question_answer 149) What is not true about alleles?
A)
Round and wrinkled form of genes are alleles of each other
done
clear
B)
Only recessive alleles expreses in hybrid
done
clear
C)
Alleles occupy same loci on homologous chromosomes
done
clear
D)
Two or more alternative forms of gene are called alfeles are allelomorphs
done
clear
View Answer play_arrow
question_answer 150) ABO blood grouping is controlled by gene I which has three alleles and show co dominance. There are six genotypes. How many phenotypes in all are possible?
A)
Three
done
clear
B)
Six
done
clear
C)
Five
done
clear
D)
Four
done
clear
View Answer play_arrow
question_answer 151) In Mendelian monohybrid cross, phenotypic ratio in\[{{F}_{2}}\]is\[3:1\]. How many types of gametes are formed in\[{{F}_{1}}-\]generation?
A)
Two types
done
clear
B)
Four types
done
clear
C)
Eight types
done
clear
D)
Only one types
done
clear
View Answer play_arrow
question_answer 152) Mendel enunciated
A)
two principles of inheritance
done
clear
B)
four principles of inheritance
done
clear
C)
five principles of inheritance
done
clear
D)
three principles of inheritance
done
clear
View Answer play_arrow
question_answer 153) Auxin was first named by
A)
C Darwin
done
clear
B)
FW Went
done
clear
C)
Alexopolus
done
clear
D)
KV Thimann
done
clear
View Answer play_arrow
question_answer 154) Which of the following inhibits\[{{O}_{2}}\]release in light phase?
A)
Zeatin
done
clear
B)
PMA
done
clear
C)
DCMU
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 155) The nitrifying bacteria that convert the ammonia to nitrates are
A)
Nitrosomonas and Nitrobacter
done
clear
B)
Azotobacter
done
clear
C)
Rhizobium sp.
done
clear
D)
Thiobacillus denitrificans
done
clear
View Answer play_arrow
question_answer 156) Phototropic curvature in Avena coleoptile is the result of uneven distribution of
A)
auxin
done
clear
B)
starch
done
clear
C)
gibberellins
done
clear
D)
phytochrome
done
clear
View Answer play_arrow
question_answer 157) Study of bakanae disease of rice led to discovery of
A)
auxins
done
clear
B)
bakery rice
done
clear
C)
Both (a) and (b)
done
clear
D)
gibberellins
done
clear
View Answer play_arrow
question_answer 158) Equipment used for the measurement of growth in plants is known as
A)
respirometer
done
clear
B)
auxanometer
done
clear
C)
atmometer
done
clear
D)
photometer
done
clear
View Answer play_arrow
question_answer 159) First transistory chemical formed by reaction between\[C{{O}_{2}}\]and RUBP is
A)
PGA
done
clear
B)
PGAL/GAP
done
clear
C)
dihydroxy acetone phosphate
done
clear
D)
2 carboxy, 3-keto, 1-5 biphosphoribotol
done
clear
View Answer play_arrow
question_answer 160) Tendrils exhibit
A)
seismonasty
done
clear
B)
geotropism
done
clear
C)
heliotropism
done
clear
D)
thigmotropism
done
clear
View Answer play_arrow
question_answer 161) The carboxylating enzyme present in the bundle sheath cells of maize leaves is
A)
RUBP carboxylase
done
clear
B)
hexokinase
done
clear
C)
carbonic anhydrase
done
clear
D)
PEP-carboxylase
done
clear
View Answer play_arrow
question_answer 162) Which of the following groups of plants are propagated through underground root?
A)
Pistia, Chrysanthemum and pineapple
done
clear
B)
Ginger, potato, onion and zamikand
done
clear
C)
Sweet potato, Asparagus, Tapioca and dahlia
done
clear
D)
Bryophyllum and Kalanchoe
done
clear
View Answer play_arrow
question_answer 163) Artificial induction of roots on stems before it is separated from the parent plant for propagation is called
A)
layering
done
clear
B)
root-stem joint
done
clear
C)
cutting
done
clear
D)
plant tissue culture
done
clear
View Answer play_arrow
question_answer 164)
Match the following columns. Column I Column II A. Zoophily 1. Pollination by birds B. Ornithophily 2. Pollination by insects C. Entomophily 3. Pollination by bats. D. Chiropterophily 4. Pollination by animals
Codes
A)
A-4, B-1, C-2, D-3
done
clear
B)
A-1, B-2, C-3, D-4
done
clear
C)
A-3, B-2, C-1, D-4
done
clear
D)
A-4, B-2, C-1, D-3
done
clear
View Answer play_arrow
question_answer 165)
Consider the following four statements I, II, III and IV and select the right option. I. The floral formula for Liliaceae is II. In vexillary aestivation, the large posterior petal is called, standard, two lateral ones are wings and two small anterior petals are termed keel. III. In pea flower the stamens are monoadelphous. IV. The floral formula for Solanaceae is
The correct statements are
A)
II and III
done
clear
B)
I and III
done
clear
C)
I and II
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 166) The scutellum observed in a grain of wheat or maize is comparable to which part of the seed in other monocotyledons?
A)
Endosperm
done
clear
B)
Cotyledon
done
clear
C)
Plumule
done
clear
D)
Aleurone layer
done
clear
View Answer play_arrow
question_answer 167) Which of the following is the scientific or botanical name of Asafoetida (Hing)?
A)
Curcuma longa
done
clear
B)
Curcuma amada
done
clear
C)
Alpinia galanga
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 168) Which one of the following classes not a representative of green-algae?
A)
Chlorophyceae
done
clear
B)
Charophyceae
done
clear
C)
Euglenophyceae
done
clear
D)
Crysophyceae
done
clear
View Answer play_arrow
question_answer 169) Which of the following codons does not select any amino acid
A)
UAG
done
clear
B)
UAA
done
clear
C)
UGA
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 170) Usually the activity of the enzymes is optimum at normal body temperature. At about\[0{}^\circ C\]the activity of the enzyme is
A)
minimum
done
clear
B)
maximum
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow