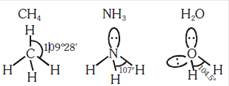
A) The H -C-H bond angle in \[C{{H}_{4}},\]the H-N- bond angle in \[\text{N}{{\text{H}}_{\text{3}}}\text{,}\]and the H-O-H bond angle in \[{{\text{H}}_{\text{2}}}\text{O}\]ar all greater than \[\text{9}{{\text{0}}^{o}}\]
B) The H-O-H bond angle in \[{{\text{H}}_{\text{2}}}\text{O}\]is larger than the H-C-H bond angle in \[\text{C}{{\text{H}}_{\text{4}}}.\]
C) The H-O-H bond angle in \[{{\text{H}}_{\text{2}}}\text{O}\]is smaller than the H-N-H bond angle in \[\text{N}{{\text{H}}_{\text{3}}}\text{.}\]
D) The H-C-H bond angle in \[\text{C}{{\text{H}}_{4}}\]is larger than the H-N-H bond angle in \[\text{N}{{\text{H}}_{\text{3}}}\text{.}\]
Correct Answer: B
Solution :
You need to login to perform this action.
You will be redirected in
3 sec
