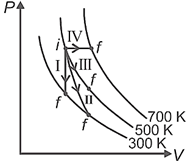
| Column-I | Column-II |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
A) \[P\to d,\,Q\to b,\,R\to a,\,S\to c\]
B) \[P\to a,\,Q\to c,\,R\to d,\,S\to b\]
C) \[P\to c,\,Q\to a,\,R\to d,\,S\to b\]
D) \[P\to c,\,Q\to d,\,R\to b,\,S\to a\]
Correct Answer: C
Solution :
Process I = Isochoric II = Adiabatic III = Isothermal IV = IsobaricYou need to login to perform this action.
You will be redirected in
3 sec
