-
question_answer1) A coil in the shape of an equilateral triangle of side (is suspended between the pole pieces of a permanent magnet such that \[\vec{B}\] is in plane of the coil. If due to a current i in the triangle a torque t acts on it, the side 2 of the triangle is:
A)
\[\frac{2}{\sqrt{3}}{{\left( \frac{\tau }{Bi} \right)}^{1/2}}\]
done
clear
B)
\[\frac{2}{\sqrt{3}}\left( \frac{\tau }{Bi} \right)\]
done
clear
C)
\[2{{\left( \frac{\tau }{\sqrt{3}\,Bi} \right)}^{1/2}}\]
done
clear
D)
\[\frac{1}{\sqrt{3}}\,\frac{\tau }{Bi}\]
done
clear
View Answer play_arrow
-
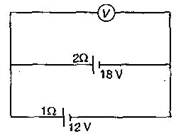
question_answer2) Two batteries, one of emf 18 V and internal resistance \[2\,\,\Omega \] and the other of emf 12 V and internal resistance \[1\,\,\Omega \], are connected as shown. The voltmeter V will record a reading of:
A)
15 V
done
clear
B)
30 V
done
clear
C)
14 V
done
clear
D)
18 V
done
clear
View Answer play_arrow
-
question_answer3) A point source emits sound equally in all directions in a non-absorbing medium. Two points P and Q are at distance of 2m and 3m respectively from the source. The ratio of the intensities of the waves at P and Q is:
A)
9 : 4
done
clear
B)
2 : 3
done
clear
C)
3 : 2
done
clear
D)
4 : 9
done
clear
View Answer play_arrow
-
question_answer4) A bomb of mass 30 kg at rest explodes into two pieces of masses 18 kg and 12 kg. The velocity of 18 kg mass is \[6\,m{{s}^{-1}}\]. The kinetic energy of the other mass is:
A)
256 J
done
clear
B)
486 J
done
clear
C)
524 J
done
clear
D)
324 J
done
clear
View Answer play_arrow
-
question_answer5) A drum of radius R and mass M, rolls down without slipping along an inclined plane of angle \[\theta \]. The frictional force:
A)
converts translational energy to rotational energy
done
clear
B)
dissipates energy as heat
done
clear
C)
decreases the rotational motion
done
clear
D)
decreases the rotational and translational motion
done
clear
View Answer play_arrow
-
question_answer6) Imagine a new planet having the same density as that of earth but it is 3 times bigger than the earth in size. If the acceleration due to gravity on the surface of earth is g and that on the surface of the new planet is g', then:
A)
\[g'=3\,g\]
done
clear
B)
\[g'=\frac{g}{9}\]
done
clear
C)
\[g'=9\,g\]
done
clear
D)
\[g'=27\,g\]
done
clear
View Answer play_arrow
-
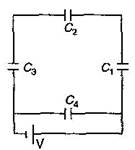
question_answer7) A network of four capacitors of capacity equal to \[{{C}_{1}}=C,\,\,{{C}_{2}}=2C,\,\,{{C}_{3}}=3C\] and \[{{C}_{4}}=4C\] are connected to a battery as shown in the figure The ratio of the charges on \[{{C}_{2}}\] an \[{{C}_{4}}\] is:
A)
\[\frac{22}{3}\]
done
clear
B)
\[\frac{3}{22}\]
done
clear
C)
\[\frac{7}{4}\]
done
clear
D)
\[\frac{4}{7}\]
done
clear
View Answer play_arrow
-
question_answer8) Which of the following circular rods, (given radius \[r\] and length \[l\]) each made of the same material and whose ends are maintained at the same temperature will conduct most heat ?
A)
\[r=2{{r}_{0}};\,l=2{{l}_{0}}\]
done
clear
B)
\[r=2{{r}_{0}};\,l={{l}_{0}}\]
done
clear
C)
\[r={{r}_{0}};\,l={{l}_{0}}\]
done
clear
D)
\[r={{r}_{0}};\,l=2{{l}_{0}}\]
done
clear
View Answer play_arrow
-
question_answer9) In the reaction \[_{1}^{2}H+\,_{1}^{3}H\,\to \,_{2}^{4}He+\,_{0}^{1}n\], if the binding energies of \[_{1}^{2}H\,,\,_{2}^{3}H\] and \[_{2}^{4}He\] are respectively a, b and c (in MeV), then the energy (in MeV) released in-this reaction is:
A)
\[c+a-b\]
done
clear
B)
\[c-a-b\]
done
clear
C)
\[a+b+c\]
done
clear
D)
\[a+b-c\]
done
clear
View Answer play_arrow
-
question_answer10)
A very long straight wire carries a current I. At the instant when a charge + Q at point P has velocity \[\vec{v}\], as shown, the force on the charge is: 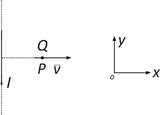
A)
opposite to ox
done
clear
B)
along ox
done
clear
C)
opposite to oy
done
clear
D)
along oy
done
clear
View Answer play_arrow
-
question_answer11) Energy levels A, B and C of a certain atom correspond to increasing values of energy i.e., \[{{E}_{A}}<{{E}_{B}}<{{E}_{C}}\]. If \[{{\lambda }_{1}},\,{{\lambda }_{2}}\] and \[{{\lambda }_{3}}\] are wavelengths of radiations corresponding to transitions C to B, B to A and C to A respectively, which of the following relations is correct?
A)
\[{{\lambda }_{3}}={{\lambda }_{1}}+{{\lambda }_{2}}\]
done
clear
B)
\[{{\lambda }_{3}}=\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
done
clear
C)
\[{{\lambda }_{1}}+{{\lambda }_{2}}+{{\lambda }_{3}}=0\]
done
clear
D)
\[\lambda _{3}^{2}=\lambda _{1}^{2}+\lambda _{2}^{2}\]
done
clear
View Answer play_arrow
-
question_answer12) The work functions for metals A, B and C are respectively 1.92 eV, 2.0 eV and 5 eV. According to Einstein's equation, the metals which will emit photoelectrons for a radiation of wavelength \[4100\overset{\text{o}}{\mathop{\text{A}}}\,\] is/are:
A)
none
done
clear
B)
A only
done
clear
C)
A and B only
done
clear
D)
all the three metals
done
clear
View Answer play_arrow
-
question_answer13) The nuclei of which one of the following pairs of nuclei are isotones?
A)
\[_{34}S{{e}^{74}},{{\,}_{31}}G{{a}^{71}}\]
done
clear
B)
\[_{42}M{{o}^{92}}\,,{{\,}_{40}}Z{{r}^{92}}\]
done
clear
C)
\[_{38}S{{r}^{84}},{{\,}_{38}}S{{r}^{86}}\]
done
clear
D)
\[_{20}C{{a}^{40}},\,{{\,}_{16}}{{S}^{32}}\]
done
clear
View Answer play_arrow
-
question_answer14)
As per this diagram a point charge +q is placed at the origin O. Work done in taking another point charge-Q from the point A [co-ordinates (0,a)] to another points [co-ordinates (a, 0)] along the straight path AB is : 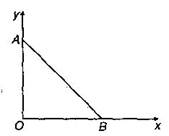
A)
zero
done
clear
B)
\[\left( \frac{-qQ}{4\pi {{\varepsilon }_{0}}}\frac{1}{{{a}^{2}}} \right)\sqrt{2}a\]
done
clear
C)
\[\left( \frac{qQ}{4\pi {{\varepsilon }_{0}}}\frac{1}{{{a}^{2}}} \right)\cdot \frac{a}{\sqrt{2}}\]
done
clear
D)
\[\left( \frac{qQ}{4\pi {{\varepsilon }_{0}}}\frac{1}{{{a}^{2}}} \right)\sqrt{2}a\]
done
clear
View Answer play_arrow
-
question_answer15)
As a result of change in the magnetic flux linked to the closed loop shown in the figure, an emf V volt is induced in the loop. The work done (joules) in taking a charge Q coulomb once along the loop is: 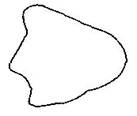
A)
QV
done
clear
B)
zero
done
clear
C)
2 QV
done
clear
D)
QV/2
done
clear
View Answer play_arrow
-
question_answer16)
For the network shown in the figure, the value of the current i is: 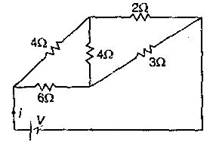
A)
\[\frac{9V}{35}\]
done
clear
B)
\[\frac{5V}{18}\]
done
clear
C)
\[\frac{5V}{9}\]
done
clear
D)
\[\frac{18V}{5}\]
done
clear
View Answer play_arrow
-
question_answer17) The circular motion of a particle with constant speed is:
A)
simple harmonic but not periodic
done
clear
B)
periodic and simple harmonic
done
clear
C)
neither periodic nor simple harmonic
done
clear
D)
periodic but not simple harmonic
done
clear
View Answer play_arrow
-
question_answer18) Particle executing simple harmonic motion of amplitude 5 cm has maximum speed of 31.4 cm/s. The frequency of its oscillation is:
A)
3 Hz
done
clear
B)
2 Hz
done
clear
C)
4 Hz
done
clear
D)
1 Hz
done
clear
View Answer play_arrow
-
question_answer19) The ratio of the dimensions of Planck's constant and that of the moment of inertia is the dimension of:
A)
frequency
done
clear
B)
velocity
done
clear
C)
angular momentum
done
clear
D)
rime
done
clear
View Answer play_arrow
-
question_answer20) Which of the following processes is reversible?
A)
Transfer of heat by radiation
done
clear
B)
Electrical heating of a nichrome wire
done
clear
C)
Transfer of heat by conduction
done
clear
D)
Isothermal compression
done
clear
View Answer play_arrow
-
question_answer21) The temperature of inversion of a thermocouple is \[{{620}^{\text{o}}}C\] and the neutral temperature is \[{{300}^{\text{o}}}C\]. What is the temperature of cold junction?
A)
\[{{20}^{\text{o}}}C\]
done
clear
B)
\[{{320}^{\text{o}}}C\]
done
clear
C)
\[-{{20}^{\text{o}}}C\]
done
clear
D)
\[{{40}^{\text{o}}}C\]
done
clear
View Answer play_arrow
-
question_answer22) A photosensitive metallic surface has work function, \[h\,{{v}_{0}}\]. If photons of energy \[2h{{v}_{0}}\] fall on this surface, the electrons come out with a maximum velocity of \[4\times {{10}^{6}}\,m/s\]. When the photon energy is increased to \[5\,h{{v}_{0}}\], then maximum velocity of photoelectrons will be:
A)
\[2\times {{10}^{6}}\,m/s\]
done
clear
B)
\[2\times {{10}^{7}}\,m/s\]
done
clear
C)
\[8\times {{10}^{5}}\,m/s\]
done
clear
D)
\[8\times {{10}^{6}}\,m/s\]
done
clear
View Answer play_arrow
-
question_answer23) Fission of nuclei is possible because the binding energy per nucleon in them:
A)
increases with mass number at high mass numbers
done
clear
B)
decreases with mass number at high mass numbers
done
clear
C)
increases with mass number at low mass numbers
done
clear
D)
decreases with mass number at low mass numbers
done
clear
View Answer play_arrow
-
question_answer24) Application of a forward bias to a p-n junction:
A)
increases the number of donors on the n-side
done
clear
B)
increases the electric field in the depletion zone
done
clear
C)
increases the potential difference across the depletion zone
done
clear
D)
widens the depletion zone
done
clear
View Answer play_arrow
-
question_answer25) The displacement \[x\] of a particle varies with time \[t\] as \[x=a{{e}^{-at}}+b{{e}^{\beta t}}\], where \[a,\,b,\,\,\alpha \] and \[\beta \] are positive constants. The velocity of the particle will:
A)
go on decreasing with time
done
clear
B)
be independent of \[\alpha \] and \[\beta \]
done
clear
C)
drop to zero when \[\alpha =\beta \]
done
clear
D)
go on increasing with time
done
clear
View Answer play_arrow
-
question_answer26)
Two charges q1 and q2 are placed 30 cm apart as shown in the figure. A third charge q3 is moved along the arc of a circle of radius 40 cm from C to D. The change in the potential energy of the system is \[\frac{{{q}_{3}}}{4\pi {{\varepsilon }_{0}}}\] k, where k is: 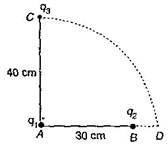
A)
\[8{{q}_{2}}\]
done
clear
B)
\[8{{q}_{1}}\]
done
clear
C)
\[6{{q}_{2}}\]
done
clear
D)
\[6{{q}_{1}}\]
done
clear
View Answer play_arrow
-
question_answer27) In any fission process the ratio \[\frac{mass\,of\,fission\,products}{mass\,of\,parent\,nucleus}\,is\,:\]
A)
less than 1
done
clear
B)
greater than 1
done
clear
C)
equal to 1
done
clear
D)
depends on the mass of parent nucleus
done
clear
View Answer play_arrow
-
question_answer28) An ideal gas heat engine operates in Carnot cycle between \[{{227}^{\text{o}}}C\] and \[{{127}^{\text{o}}}C\]. It absorbs \[6\times {{10}^{4}}\] cal of heat at higher temperature. Amount of heat converted to work is:
A)
\[2.4\times {{10}^{4}}\,cal\]
done
clear
B)
\[6\times {{10}^{4}}\,cal\]
done
clear
C)
\[1.2\times {{10}^{4}}\,cal\]
done
clear
D)
\[4.8\times {{10}^{4}}\,cal\]
done
clear
View Answer play_arrow
-
question_answer29) If a vector \[2\hat{i}+3\hat{j}+8\hat{k}\] is perpendicular to the vector \[4\hat{j}-4\hat{i}+\alpha \hat{k},\] then the value of a is:
A)
-1
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[-\frac{1}{2}\]
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer30) Zener diode is used for:
A)
producing oscillations in an oscillator
done
clear
B)
amplification
done
clear
C)
stabilization
done
clear
D)
rectification
done
clear
View Answer play_arrow
-
question_answer31)
A force F acting on an object varies with distance \[x\] as shown here. The force is in N and \[x\] is in m. The work done by the force in moving the object from x = 0 to x = 6 m is: 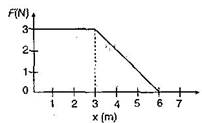
A)
4.5 J
done
clear
B)
13.5 J
done
clear
C)
9.0 J
done
clear
D)
18.0 J
done
clear
View Answer play_arrow
-
question_answer32) A stone tied to the end of a string of 1 m long is whirled in a horizontal circle with a constant speed. If the stone makes 22 revolutions in 44 s, what is the magnitude and direction of acceleration of the stone?
A)
\[\frac{\pi }{2}\] \[m{{s}^{-2}}\]and direction along the radius towards the centre
done
clear
B)
\[{{\pi }^{2}}\,m{{s}^{-2}}\]and direction along the radius away from centre
done
clear
C)
\[{{\pi }^{2}}\,m{{s}^{-2}}\] and direction along the radius towards the centre
done
clear
D)
\[{{\pi }^{2}}\,m{{s}^{-2}}\] and direction along the tangent to the circle
done
clear
View Answer play_arrow
-
question_answer33) If the magnetic dipole moment of an atom of diamagnetic material, paramagnetic material and ferromagnetic material are denoted by \[{{\mu }_{d}},{{\mu }_{p}}\] and \[{{\mu }_{f}}\]respectively, then:
A)
\[{{\mu }_{d}}\ne 0\,\,and\,\,{{\mu }_{f}}\ne 0\]
done
clear
B)
\[{{\mu }_{p}}=0\,and\,{{\mu }_{f}}\ne 0\]
done
clear
C)
\[{{\mu }_{d}}=0\,\,and\,\,{{\mu }_{p}}\ne 0\]
done
clear
D)
\[{{\mu }_{d}}\ne 0\,and\,{{\mu }_{p}}=0\]
done
clear
View Answer play_arrow
-
question_answer34)
In a circuit, L, C and R are connected in series with an alternating voltage source of frequency f. The current leads the voltage by 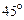 . The value of C is:
. The value of C is:
A)
\[\frac{1}{2\pi f\,(2\pi fL+R)}\]
done
clear
B)
\[\frac{1}{\pi f\,(2\pi fL+R)}\]
done
clear
C)
\[\frac{1}{2\pi f\,(2\pi fL-R)}\]
done
clear
D)
\[\frac{1}{\pi f\,(2\pi fL-R)}\]
done
clear
View Answer play_arrow
-
question_answer35) The angular resolution of a 10 cm diameter telescope at a wavelength of \[5000\overset{\text{o}}{\mathop{\text{A}}}\,\] is of the -order of:
A)
\[{{10}^{6}}rad\]
done
clear
B)
\[{{10}^{-2}}rad\]
done
clear
C)
\[{{10}^{-4}}\,rad\]
done
clear
D)
\[{{10}^{-6}}rad\]
done
clear
View Answer play_arrow
-
question_answer36) Two vibrating tuning forks produce progressive waves given by \[{{y}_{1}}=4\sin 500\,\pi t\] and \[{{y}_{2}}=2\sin \,506\,\pi t\]. Number of beats produced per minute is:
A)
360
done
clear
B)
180
done
clear
C)
3
done
clear
D)
60
done
clear
View Answer play_arrow
-
question_answer37) When a wire of uniform cross-section a, length \[l\] and resistance R is bent into a complete circle, resistance between two of diametrically opposite points will be:
A)
\[\frac{R}{4}\]
done
clear
B)
\[\frac{R}{8}\]
done
clear
C)
4 R
done
clear
D)
\[\frac{R}{2}\]
done
clear
View Answer play_arrow
-
question_answer38) Carbon, silicon and germanium atoms have four valence electrons each. Their valence and conduction bands are separated by energy band gaps represented by \[{{({{E}_{g}})}_{C}},\] \[{{({{E}_{g}})}_{Si}}\] and \[({{E}_{g}}){{G}_{Ge}}\] respectively. Which one of the following relationships is true in their case?
A)
\[{{({{E}_{g}})}_{C}}>{{({{E}_{g}})}_{Si}}\]
done
clear
B)
\[{{({{E}_{g}})}_{C}}={{({{E}_{g}})}_{Si}}\]
done
clear
C)
\[{{({{E}_{g}})}_{C}}<({{E}_{g}})Ge\]
done
clear
D)
\[{{({{E}_{g}})}_{C}}<{{({{E}_{g}})}_{Si}}\]
done
clear
View Answer play_arrow
-
question_answer39) If \[{{\lambda }_{v}},\,{{\lambda }_{x}}\,\]and \[{{\lambda }_{m}}\]represent the wavelengths of visible light, X-rays and microwaves respectively, then:
A)
\[{{\lambda }_{m}}>{{\lambda }_{x}}>{{\lambda }_{v}}\]
done
clear
B)
\[{{\lambda }_{v}}>{{\lambda }_{m}}>{{\lambda }_{x}}\]
done
clear
C)
\[{{\lambda }_{m}}>{{\lambda }_{v}}>{{\lambda }_{x}}\]
done
clear
D)
\[{{\lambda }_{v}}>{{\lambda }_{x}}>{{\lambda }_{m}}\]
done
clear
View Answer play_arrow
-
question_answer40) Two boys are standing at the ends A and B of a ground, where AB = a. The boy at B starts running in a direction perpendicular to AB with velocity \[{{v}_{1}}\]. The boy at A starts running simultaneously with velocity v and catches the other boy in a time t, where t is:
A)
\[\frac{a}{\sqrt{{{v}^{2}}+v_{1}^{2}}}\]
done
clear
B)
\[\sqrt{\frac{{{a}^{2}}}{{{v}^{2}}-v_{1}^{2}}}\]
done
clear
C)
\[\frac{a}{(v-{{v}_{1}})}\]
done
clear
D)
\[\frac{a}{(v+{{v}_{1}})}\]
done
clear
View Answer play_arrow
-
question_answer41) A 5-A fuse wire can withstand a maximum power of 1 W in circuit. The resistance of the fuse wire is:
A)
0.2 \[\Omega \]
done
clear
B)
5 \[\Omega \]
done
clear
C)
0.4 \[\Omega \]
done
clear
D)
0.04 \[\Omega \]
done
clear
View Answer play_arrow
-
question_answer42) Two bodies have their moments of inertia \[l\] and \[2\,l\] respectively about their axis of rotation. If their kinetic energies of rotation are equal, their angular momenta will be in the ratio:
A)
1 : 2
done
clear
B)
\[\sqrt{2}\,:1\]
done
clear
C)
2 : 1
done
clear
D)
\[1:\sqrt{2}\]
done
clear
View Answer play_arrow
-
question_answer43) An electron moves in a circular orbit with a uniform speed v. It produces a magnetic field B, at the centre of the circle. The radius of the circle is proportional to:
A)
\[\frac{B}{v}\]
done
clear
B)
\[\frac{v}{B}\]
done
clear
C)
\[\sqrt{\frac{v}{B}}\]
done
clear
D)
\[\sqrt{\frac{B}{v}}\]
done
clear
View Answer play_arrow
-
question_answer44) Choose the only false statement from the following:
A)
Substances with energy gap of the order of 10 eV are insulators
done
clear
B)
The conductivity of a semiconductor increases with increases in temperature
done
clear
C)
In conductors the valence and conduction bands may overlap
done
clear
D)
The resistivity of a semiconductor increases with increase in temperature
done
clear
View Answer play_arrow
-
question_answer45) If the angle between the vectors \[\vec{A}\] and \[\vec{B}\] is \[\theta \], the value of the product \[(\vec{B}\times \vec{A}).\vec{A}\] is equal to:
A)
\[B{{A}^{2}}\cos \theta \]
done
clear
B)
\[B{{A}^{2}}\sin \theta \]
done
clear
C)
\[B{{A}^{2}}\sin \theta \cos \theta \]
done
clear
D)
zero
done
clear
View Answer play_arrow
-
question_answer46) The moment of inertia of a uniform circular disc of radius R and mass M about an axis passing from the edge of the disc and normal to the disc is:
A)
\[\frac{1}{2}\,M{{R}^{2}}\]
done
clear
B)
\[M{{R}^{2}}\]
done
clear
C)
\[\frac{7}{2}M{{R}^{2}}\]
done
clear
D)
\[\frac{3}{2}M{{R}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer47) Copper has face-centered cubic (fcc) lattice with interatomic spacing equal to \[2.54\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. The value of lattice constant for this lattice is:
A)
\[1.27\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[5.08\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[2.54\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[3.59\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
-
question_answer48) The total energy of an electron in the first excited state of hydrogen is about -3.4 eV. Its kinetic energy in this state is:
A)
-3.4eV
done
clear
B)
-6.8eV
done
clear
C)
6.8 eV
done
clear
D)
3.4 Ev
done
clear
View Answer play_arrow
-
question_answer49) For a satellite moving in an orbit around the earth, the ratio of kinetic energy to potential energy is:
A)
2
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{1}{\sqrt{2}}\]
done
clear
D)
\[\sqrt{2}\]
done
clear
View Answer play_arrow
-
question_answer50) A ball is thrown vertically upward. It has a speed of 10 m/s when it has reached one half of its maximum height. How high does the ball rise? (Taking \[g=10\,m/{{s}^{2}}\])
A)
15 m
done
clear
B)
10 m
done
clear
C)
20 m
done
clear
D)
5 m
done
clear
View Answer play_arrow
-
question_answer51) Which amongst the following is the most stable carbocation?
A)
\[\begin{align} & C{{H}_{3}}-\underset{|}{\mathop{\overset{+}{\mathop{C}}\,}}\,-H \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}} \\ \end{align}\]
done
clear
B)
\[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}} \\ & C{{H}_{3}}-\underset{|}{\mathop{\overset{|}{\mathop{{{C}^{+}}}}\,}}\, \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}} \\ \end{align}\]
done
clear
C)
\[\overset{+}{\mathop{C}}\,{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer52) Products of the following reaction: \[C{{H}_{3}}C\equiv C.C{{H}_{2}}C{{H}_{3}}\underset{(2)\,Hydrolysis}{\mathop{\xrightarrow{(1)\,{{O}_{3}}}}}\,\,......are\,:\]
A)
\[C{{H}_{3}}CHO+C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
B)
\[C{{H}_{3}}COOH+C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}COOH+HOOC.C{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COOH+C{{O}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer53) At \[{{25}^{\text{o}}}C,\] the dissociation constant of a base, BOH, is \[1.0\times {{10}^{-12}}\]. The concentration of hydroxyl ions in 0.01 M aqueous solution of the base would be:
A)
\[2.0\times {{10}^{-6}}\,mol\,{{L}^{-1}}\]
done
clear
B)
\[1.0\times {{10}^{-5}}\,mol\,{{L}^{-1}}\]
done
clear
C)
\[1.0\times {{10}^{-6}}\,mol\,{{L}^{-1}}\]
done
clear
D)
\[1.0\times {{10}^{-7}}\,mol\,{{L}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer54) Which one of the following pairs represents stereoisomerism?
A)
Chain isomerism and rotational isomerism
done
clear
B)
Structural isomerism and geometric isomerism
done
clear
C)
Linkage isomerism and geometric isomerism
done
clear
D)
Optical isomerism and geomertric isomerism
done
clear
View Answer play_arrow
-
question_answer55)
Aniline in a set of reactions yielded a product D. 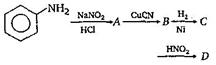 The structure of die product D would be:
The structure of die product D would be:
A)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}>{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}NHC{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}NHOH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
-
question_answer56) The correct order in which the O?O bond length increases in the following is:
A)
\[{{H}_{2}}{{O}_{2}}<{{O}_{2}}<{{O}_{3}}\]
done
clear
B)
\[{{O}_{3}}<{{H}_{2}}{{O}_{2}}<{{O}_{2}}\]
done
clear
C)
\[{{O}_{2}}<{{O}_{3}}<{{H}_{2}}{{O}_{2}}\]
done
clear
D)
\[{{O}_{2}}<{{H}_{2}}{{O}_{2}}<{{O}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer57) The mass of carbon anode consumed (giving only carbondioxide) in the production of 270 kg of aluminium metal from bauxite by the Hall process is: (Atomic mass Al = 27)
A)
180 kg
done
clear
B)
270 kg
done
clear
C)
540 kg
done
clear
D)
90 kg
done
clear
View Answer play_arrow
-
question_answer58) In a set of reactions, acetic acid yielded a product D. \[C{{H}_{3}}COOH\xrightarrow[{}]{SOC{{l}_{2}}}A\underset{Anhyd.\,AlC{{l}_{3}}}{\mathop{\xrightarrow{Benzene}}}\,B\xrightarrow[{}]{HCN}C\xrightarrow[{}]{HOH}D\] The structure of D would be:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer59) The cell membranes are mainly composed of:
A)
carbohydrates
done
clear
B)
proteins
done
clear
C)
phospholipids
done
clear
D)
fats
done
clear
View Answer play_arrow
-
question_answer60)
The major organic product formed from the following reaction: 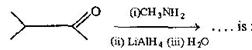
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer61) The number of moles of \[KMn{{O}_{4}}\] reduced by one mole of KI in alkaline medium is:
A)
one fifth
done
clear
B)
five
done
clear
C)
one
done
clear
D)
two
done
clear
View Answer play_arrow
-
question_answer62) Which of the following molecules has trigonal planar geometry?
A)
\[I{{F}_{3}}\]
done
clear
B)
\[PC{{l}_{3}}\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[B{{F}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer63) The aqueous solution containing which one of the following ions will be colourless? (Atomic no. : Sc = 21, Fe = 26, Ti = 22, Mn = 25)
A)
\[S{{c}^{3+}}\]
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[T{{i}^{3+}}\]
done
clear
D)
\[M{{n}^{2+}}\]
done
clear
View Answer play_arrow
-
question_answer64) Four successive members of the first row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionization enthalpy?
A)
Vanadium (Z = 23)
done
clear
B)
Chromium (Z = 24)
done
clear
C)
Iron (2 = 26)
done
clear
D)
Manganese (Z = 25)
done
clear
View Answer play_arrow
-
question_answer65) Which one of the following compounds is most acidic?
A)
\[Cl-C{{H}_{2}}-C{{H}_{2}}-OH\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer66) A reaction occurs spontaneously if:
A)
\[T\Delta S<\Delta H\]and both \[\Delta H\] and \[\Delta S\] are +ve
done
clear
B)
\[T\Delta S>\Delta H\] and both \[\Delta H\] and \[\Delta S\] are +ve
done
clear
C)
\[T\Delta S=\Delta H\]and both \[\Delta H\] and \[\Delta S\] are +ve
done
clear
D)
\[T\Delta S>\Delta H\] and \[\Delta H\] is + ve and \[\Delta S\] is ?ve
done
clear
View Answer play_arrow
-
question_answer67)
The monomer of the polymer: 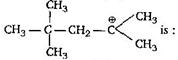
A)
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}C=C{{(C{{H}_{3}})}_{2}}\]
done
clear
C)
\[C{{H}_{3}}CH=CH.C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer68) The correct sequence of increasing covalent character is represented by:
A)
\[LiCl<NaCl<BeC{{l}_{2}}\]
done
clear
B)
\[BeC{{l}_{2}}<NaCl<LiCl\]
done
clear
C)
\[NaCl<LiCl<BeC{{l}_{2}}\]
done
clear
D)
\[BeC{{l}_{2}}<LiCl<NaCl\]
done
clear
View Answer play_arrow
-
question_answer69) What is the correct relationship between the pHs of isomolar solutions of sodium oxide \[(p{{H}_{1}})\], sodium sulphide \[(p{{H}_{2}})\] sodium selenide \[(p{{H}_{3}})\] and sodium telluride \[(p{{H}_{4}})\]?
A)
\[p{{H}_{1}}>p{{H}_{2}}\approx p{{H}_{3}}>p{{H}_{4}}\]
done
clear
B)
\[p{{H}_{1}}<p{{H}_{2}}<p{{H}_{3}}<p{{H}_{4}}\]
done
clear
C)
\[p{{H}_{1}}<p{{H}_{2}}<p{{H}_{3}}\approx p{{H}_{4}}\]
done
clear
D)
\[p{{H}_{1}}>p{{H}_{2}}>p{{H}_{3}}>p{{H}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer70) Which of the following pairs of a chemical reaction is certain to result in a spontaneous reaction?
A)
Exothermic and decreasing disorder
done
clear
B)
Endothermic and increasing disorder
done
clear
C)
Exothermic and increasing disorder
done
clear
D)
Endothermic and decreasing disorder
done
clear
View Answer play_arrow
-
question_answer71) The vapour pressure of two liquids P and Q are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 moles of P and 2 moles of Q would be:
A)
140 torr
done
clear
B)
20 torr
done
clear
C)
68 torr
done
clear
D)
72 torr
done
clear
View Answer play_arrow
-
question_answer72) Which one of the following alkenes will react faster with H2 under catalytic hydrogenation conditions?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer73) For a first order reaction \[A\xrightarrow[{}]{{}}B\,\], the reaction rate at reactant concentration of 0.01 M is found to be \[2.0\times {{10}^{-5}}\,mol\,{{L}^{-1}}\,{{s}^{-1}}\]. The half life period of the reaction is:
A)
220 s
done
clear
B)
30 s
done
clear
C)
300 s
done
clear
D)
347 s
done
clear
View Answer play_arrow
-
question_answer74) Which of the following is the electron deficient molecule?
A)
\[{{B}_{2}}{{H}_{6}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[P{{H}_{3}}\]
done
clear
D)
\[Si{{H}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer75) A nuclide of an alkaline earth metal undergoes radioactive decay by emission of three \[\alpha \]-particles in succession. The group of the periodic table to which the resulting daughter element would belong is:
A)
Group 14
done
clear
B)
Group 16
done
clear
C)
Group 4
done
clear
D)
Group 6
done
clear
View Answer play_arrow
-
question_answer76) The surface tension of which of the following liquid is maximum?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
C)
\[C{{H}_{3}}OH\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
-
question_answer77) The absolute enthalpy of neutralisation of the reaction: MgO(s) + 2HCI (aq) \[\to \]\[MgC{{l}_{2}}(ag)+{{H}_{2}}O(l)\] will be:
A)
less than \[-57.33\,kJ\,mo{{l}^{-1}}\]
done
clear
B)
\[-57.33\,kJ\,mo{{l}^{-1}}\]
done
clear
C)
greater than \[-57.33\,kJ\,mo{{l}^{-1}}\]
done
clear
D)
\[-57.33\,kJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer78) Which one of the following forms micelles in aqueous solution above certain concentration?
A)
Urea
done
clear
B)
Dodecyl trimethyl ammonium chloride
done
clear
C)
Pyridinium chloride
done
clear
D)
Glucose
done
clear
View Answer play_arrow
-
question_answer79) Electrolytic reduction of nitrobenzene in weakly acidic medium gives:
A)
aniline
done
clear
B)
nitrosobenzene
done
clear
C)
N-phenylhydroxylamine
done
clear
D)
p-hydroxyaniline
done
clear
View Answer play_arrow
-
question_answer80) Equilibrium constants \[{{K}_{1}}\] and \[{{K}_{2}}\] for the following equilibria: \[{{N}_{2}}(g)+\frac{1}{2}{{O}_{2}}\,N{{O}_{2}}(g)\,and\,\] \[2N{{O}_{2}}(g)\,2NO(g)+{{O}_{2}}(g)\] are related as:
A)
\[{{K}_{2}}=\frac{1}{{{K}_{1}}}\]
done
clear
B)
\[{{K}_{2}}=K_{1}^{2}\]
done
clear
C)
\[{{K}_{2}}=\frac{{{K}_{1}}}{2}\]
done
clear
D)
\[{{K}_{2}}=\frac{1}{K_{1}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer81) Which of the following would have a permanent dipole moment?
A)
\[B{{F}_{3}}\]
done
clear
B)
\[Si{{F}_{4}}\]
done
clear
C)
\[S{{F}_{4}}\]
done
clear
D)
\[Xe{{F}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer82) Which of the following undergoes nucleophilic substitution exclusively by \[{{S}_{N}}1\] mechanism?
A)
Benzyl chloride
done
clear
B)
Ethyl chloride
done
clear
C)
Chlorobenzene
done
clear
D)
Isopropyl chloride
done
clear
View Answer play_arrow
-
question_answer83) The rate of reaction between two reactants A and B decreases by a factor of 4, if die concentration of reactant B is doubled. The order of this reaction with respect to reactant B is:
A)
-1
done
clear
B)
-2
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow
-
question_answer84) In a face-centered cubic lattice, a unit cell is shared equally by how many unit cells?
A)
8
done
clear
B)
4
done
clear
C)
2
done
clear
D)
6
done
clear
View Answer play_arrow
-
question_answer85) A solution of urea (mol. mass \[56\,g\,mo{{l}^{-1}}\]) boils at 100.18°C at the atmospheric pressure. If \[{{k}_{f}}\] and \[{{k}_{b}}\] for water are 1.86 and \[0.512K\,kg\,mo{{l}^{-1}}\] respectively, die above solution will freeze at:
A)
-6.54°C
done
clear
B)
6.54°C
done
clear
C)
0.654°C
done
clear
D)
- 0.654°C
done
clear
View Answer play_arrow
-
question_answer86) Which functional group -participates in disulphide bond formation in proteins?
A)
Thiolactone
done
clear
B)
Thiol
done
clear
C)
Thioether
done
clear
D)
Thioester
done
clear
View Answer play_arrow
-
question_answer87) Which one of the following is an inner orbital complex as well as diamagnetic in behaviour? (Atomic no. : Zn = 30, Cr = 24, Co = 27, Ni = 28)
A)
\[{{[Zn\,{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
done
clear
B)
\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
D)
\[{{[Ni\,{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
-
question_answer88)
The chirality of the compound 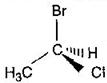 is:
is:
A)
R
done
clear
B)
S
done
clear
C)
Z
done
clear
D)
E
done
clear
View Answer play_arrow
-
question_answer89) \[{{H}_{2}}S\] gas when passed through a solution of cations containing HCl precipitates the cations of second group of qualitative analysis but not those belonging to the fourth group. It is because:
A)
presence of HCl decreases the sulphide ion concentration
done
clear
B)
presence of HCl increases the sulphide ion concentration
done
clear
C)
solubility product of group II sulphides is more than that of group IV sulphides
done
clear
D)
sulphides of group IV cations are unstable in HCl
done
clear
View Answer play_arrow
-
question_answer90) Which one of the following oxides is expected to exhibit paramagnetic behaviour?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[Cl{{O}_{2}}\]
done
clear
D)
\[Si{{O}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer91) Which one of the following is expected to exhibit optical isomerism? (en = ethylenediamine)
A)
\[cis-[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
B)
\[trans-[Co{{(en)}_{2}}C{{l}_{2}}]\]
done
clear
C)
trans-\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
D)
\[cis-[Co{{(en)}_{2}}C{{l}_{2}}]\]
done
clear
View Answer play_arrow
-
question_answer92) The energy of second Bohr orbit of the hydrogen atom is \[-328\,\,mJ\,mo{{l}^{-1}};\] hence the energy of fourth Bohr orbit would be:
A)
\[-41\,\,kJ\,mo{{l}^{-1}}\]
done
clear
B)
\[-1312\,kJ\,mo{{l}^{-1}}\]
done
clear
C)
\[-164\,kJ\,mo{{l}^{-1}}\]
done
clear
D)
\[-82\,kJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer93) The correct order of acid strength is:
A)
\[HClO<HCl{{O}_{2}}<HCl{{O}_{3}}<HCl{{O}_{4}}\]
done
clear
B)
\[HCl{{O}_{4}}<HClO<HCl{{O}_{2}}<HCl{{O}_{3}}\]
done
clear
C)
\[HCl{{O}_{2}}<HCl{{O}_{3}}<HCl{{O}_{4}}<HClO\]
done
clear
D)
\[HCl{{O}_{4}}<HCl{{O}_{3}}<HCl{{O}_{2}}<HClO\]
done
clear
View Answer play_arrow
-
question_answer94) The main reason for larger number of oxidation states exhibited by the actinides than the corresponding lanthanides, is:
A)
lesser energy difference between \[5f\] and 6d orbitals than between \[4f\] and 5d orbitals
done
clear
B)
larger atomic size of actinides than the lanthanides
done
clear
C)
more energy difference between 5f and 6d orbitals than between 4f and 5d orbitals
done
clear
D)
greater reactive nature of the actinides than the lanthanides
done
clear
View Answer play_arrow
-
question_answer95) Names of some compounds are given. Which one is not correct in IUPAC system?
A)
\[\underset{\text{3-methyl-2 butanol}}{\mathop{C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}}}\,\]
done
clear
B)
\[\underset{\text{4-methyl-2-pentyne}}{\mathop{C{{H}_{3}}-C\equiv C}}\,-CH{{(C{{H}_{3}})}_{2}}\]
done
clear
C)
\[\underset{\text{2-ethyl-3 methyl-but-1-ene}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ C{{H}_{2}} \end{smallmatrix}}{\mathop{C}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}}}\,\]
done
clear
D)
\[\underset{\text{3-methyl-4 ehtyl heptane}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{2}}C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}C{{H}_{3}}}}\,\]
done
clear
View Answer play_arrow
-
question_answer96) Which one of the following arrangements represents the correct order of electron gain enthalpy (with negative sign) of the given atomic species?
A)
Cl < F < S < O
done
clear
B)
O < S < F < Cl
done
clear
C)
S < O < Cl < F
done
clear
D)
F < Cl < O < S
done
clear
View Answer play_arrow
-
question_answer97) A solution has a 1 : 4 mole ratio of pentane to hexane. The vapour pressure of the pure hydrocarbons at 20°C are 440 mm of Hg for pentane and 120 mm of Hg for hexane. The mole fraction of pentarie in the vapour phase would be:
A)
0.549
done
clear
B)
0.200
done
clear
C)
0.786
done
clear
D)
0.478
done
clear
View Answer play_arrow
-
question_answer98) 4.5 g of aluminium (at. mass 27 amu) is deposited at cathode from \[A{{l}^{3+}}\] solution by a certain quantity of electric charge. The volume of hydrogen produced at STP from \[{{H}^{+}}\] ions in solution by the same quantity of electric charge will be:
A)
22.4 L
done
clear
B)
44.8 L
done
clear
C)
5.6 L
done
clear
D)
11.2 L
done
clear
View Answer play_arrow
-
question_answer99) The best method for the separation of naphthalene and benzoic acid from their mixture is:
A)
chromatography
done
clear
B)
crystallization
done
clear
C)
distillation
done
clear
D)
sublimation
done
clear
View Answer play_arrow
-
question_answer100) The mole fraction of the solute in one molal aqueous solution is:
A)
0.027
done
clear
B)
0.036
done
clear
C)
0.018
done
clear
D)
0.009
done
clear
View Answer play_arrow
-
question_answer101) The main organelle involved in modification and routing of newly synthesized proteins to their destinations is:
A)
mitochondria
done
clear
B)
endoplasmic reticulum
done
clear
C)
lysosome
done
clear
D)
chloroplast
done
clear
View Answer play_arrow
-
question_answer102) There are two opposing views about origin of Modern man. According to one view Homo erectus in Asia were the ancestors of modern man. A study of variations of DNA however suggested African origin of Modem man. What kind of observation on DNA variation could suggest this?
A)
Greater variation in Africa than in Asia
done
clear
B)
Variation only in Asia and no variation in Africa
done
clear
C)
Greater variation in Asia than in Africa
done
clear
D)
Similar variation in Africa and Asia
done
clear
View Answer play_arrow
-
question_answer103) The world's highly prized wool yielding 'Pashmina' breed is:
A)
sheep
done
clear
B)
goat
done
clear
C)
goat-sheep cross
done
clear
D)
Kashmir sheep-Afghan sheep cross
done
clear
View Answer play_arrow
-
question_answer104) Grey crescent is the area:
A)
at the point of entry of sperm into ovum
done
clear
B)
just opposite to the site of entry of sperm into ovum
done
clear
C)
at the animal pole
done
clear
D)
at the vegetal pole
done
clear
View Answer play_arrow
-
question_answer105) Photosynthesis in \[{{C}_{4}}\] plants is relatively less limited by atmospheric \[C{{O}_{2}}\] levels because:
A)
four carbon acids are the primary initial \[C{{O}_{2}}\] fixation products
done
clear
B)
the primary fixation of \[C{{O}_{2}}\] is mediated via PEP carboxylase
done
clear
C)
effective pumping of \[C{{O}_{2}}\] into bundle sheath cells
done
clear
D)
RUBISCO in \[{{C}_{4}}\] plants has higher affinity for \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer106) At what stage of the cell cycle are histone proteins synthesized in a eukaryotic cell?
A)
During entire prophase
done
clear
B)
During telophase
done
clear
C)
During S-phase
done
clear
D)
During \[{{G}_{2}}\] stage of prophase
done
clear
View Answer play_arrow
-
question_answer107) There exists a dose association between the alga and the fungus within a lichen. The fungus:
A)
fixes the atmospheric nitrogen for the alga
done
clear
B)
provides protection, anchorage and absorption for the alga
done
clear
C)
provides food for the alga
done
clear
D)
releases oxygen for the alga
done
clear
View Answer play_arrow
-
question_answer108) For retting of jute the fermenting microbe used is:
A)
Helkobactor pylori
done
clear
B)
Mcthophilic bacteria
done
clear
C)
Streptococcus lactin
done
clear
D)
Butyric acid bacteria
done
clear
View Answer play_arrow
-
question_answer109) A student wishes to study the cell structure under a light microscope having 10X eyepiece and 45X objective. He should illuminate the object by which one of the following colours of light so as to get the best possible resolution?
A)
Yellow
done
clear
B)
Green
done
clear
C)
Blue
done
clear
D)
Red
done
clear
View Answer play_arrow
-
question_answer110) The net pressure gradient that causes the fluid to filter out of the glomeruli into the capsule is:
A)
20 mm Hg
done
clear
B)
75 mm Hg
done
clear
C)
30 mm Hg
done
clear
D)
50 mm Hg
done
clear
View Answer play_arrow
-
question_answer111) At which latitude, heat gain through insolation approximately equals heat loss through terrestrial radiation?
A)
\[{{66}^{o}}\] North and South
done
clear
B)
\[22\frac{1}{2}{{\,}^{o}}\] North and South
done
clear
C)
\[{{40}^{o}}\] North and South
done
clear
D)
\[42\frac{1}{2}{{\,}^{o}}\] North and South
done
clear
View Answer play_arrow
-
question_answer112) A man and a woman, who do not show any apparent signs of a certain inherited disease, have seven children (2 daughters and 5 sons). Three of the sons suffer from the given disease but none of the daughters are affected. Which of the following mode of inheritance do you suggest for this disease?
A)
Autosomal dominant
done
clear
B)
Sex-linked dominant
done
clear
C)
Sex-limited recessive
done
clear
D)
Sex-linked recessive
done
clear
View Answer play_arrow
-
question_answer113) In Ornithine cycle, which of the following wastes are removed from the blood?
A)
Urea and urine
done
clear
B)
Ammonia and urea
done
clear
C)
\[C{{O}_{2}}\] and ammonia
done
clear
D)
\[C{{O}_{2}}\] and urea
done
clear
View Answer play_arrow
-
question_answer114) Telomerase is an enzyme which is a:
A)
repetitive DNA
done
clear
B)
RNA
done
clear
C)
simple protein
done
clear
D)
ribonucleoprotein
done
clear
View Answer play_arrow
-
question_answer115) During transcription holoenzyme RNA polymerase binds to a DNA sequence and the DNA assumes a saddle like structure at that point. What is that sequence called?
A)
CAAT box
done
clear
B)
GGTT box
done
clear
C)
AAAT box
done
clear
D)
TATA box
done
clear
View Answer play_arrow
-
question_answer116) Centromere is required for:
A)
transcription
done
clear
B)
crossing over
done
clear
C)
cytoplasmic cleavage
done
clear
D)
movement of chromosomes towards poles
done
clear
View Answer play_arrow
-
question_answer117) Damage to thymus in a child may lead to:
A)
a reduction in haemoglobin content of blood
done
clear
B)
a reduction in stem cell production
done
clear
C)
loss of antibody mediated immunity
done
clear
D)
loss of cell mediated immunity
done
clear
View Answer play_arrow
-
question_answer118) Prolonged liberal irrigation of agricultural fields is likely to create the problem of:
A)
acidity
done
clear
B)
aridity
done
clear
C)
metal toxicity
done
clear
D)
salinity
done
clear
View Answer play_arrow
-
question_answer119) Chlorophyll in chloroplasts is located in:
A)
grana
done
clear
B)
pyrenoid
done
clear
C)
stroma
done
clear
D)
both (a) and (c)
done
clear
View Answer play_arrow
-
question_answer120) Three crops that contribute maximum to global food grain production are:
A)
wheat, rice and maize
done
clear
B)
wheat, maize and sorghum
done
clear
C)
rice, maize and sorghum
done
clear
D)
wheat, rice and barley
done
clear
View Answer play_arrow
-
question_answer121) Genes for cytoplasmic male sterility in plants are generally located in:
A)
mitochondrial genome
done
clear
B)
cytosol
done
clear
C)
chloroplast genome
done
clear
D)
nuclear genome
done
clear
View Answer play_arrow
-
question_answer122) Which one of the following hydrolyses internal phosphodiester bonds in a polynucleotide chain?
A)
Lipase
done
clear
B)
Exonuclease
done
clear
C)
Endonuclease
done
clear
D)
Protease
done
clear
View Answer play_arrow
-
question_answer123) Match items in column-I with those in Column-II. Column-I Column-II A. Peritrichous flagellation 1. Ginkgo B. Living fossil 2. Macrocystes C. Khizophore 3. Escherichia coli D. Smallest flowering plant 4. SelagmeHa E. Largest perennial alga 5. Wolffia Select the correct answer from the following:
A)
Codes: A B C D E 2 1 3 4 5
done
clear
B)
5 3 2 5 1
done
clear
C)
1 2 5 3 2
done
clear
D)
3 1 4 5 2
done
clear
View Answer play_arrow
-
question_answer124) The name of Norman Borlaug is associated with:
A)
Green revolution
done
clear
B)
Yellow revolution
done
clear
C)
White revolution
done
clear
D)
Blue revolution
done
clear
View Answer play_arrow
-
question_answer125) G-6-P dehydrogenase deficiency is associated with haemolysis of:
A)
lymphocytes
done
clear
B)
RBCs
done
clear
C)
platelets
done
clear
D)
leucocytes
done
clear
View Answer play_arrow
-
question_answer126) In which one pair both the plants can be vegetatively propagated by leaf pieces?
A)
Bryophyllum and Kalanchoe
done
clear
B)
Chrysanthemum and Agave
done
clear
C)
Agave and Kalanchoe
done
clear
D)
Asparagus and Bryophyllum
done
clear
View Answer play_arrow
-
question_answer127) According to IUCN red list what is the status of red Panda (Athurus fulgens)?
A)
Vulnerable species
done
clear
B)
Critically endangered species
done
clear
C)
Extinct species
done
clear
D)
Endangered species
done
clear
View Answer play_arrow
-
question_answer128) Which of the following substances, if introduce in the blood stream, would cause coagulation, at the site of its introduction?
A)
Fibrinogen
done
clear
B)
Prothrombin
done
clear
C)
Heparin
done
clear
D)
Thromboplastin
done
clear
View Answer play_arrow
-
question_answer129) E. coli cells with a mutated Z gene of the lac operon cannot grow in medium containing only lactose as the source of energy because:
A)
in the presence of glucose, E. coli cells do not utilize lactose
done
clear
B)
they cannot transport lactose from the medium into the cell
done
clear
C)
the lac operon is constitutively active in these cells
done
clear
D)
they cannot synthesize functional \[\beta \]-galactosidase
done
clear
View Answer play_arrow
-
question_answer130) Top-shaped multiciliate male gametes and the mature seed which bears only one embryo with two cotyledons, are characteristic features of:
A)
polypetalous angiosperms
done
clear
B)
gamopetalous angiosperms
done
clear
C)
conifers
done
clear
D)
cycads
done
clear
View Answer play_arrow
-
question_answer131) Production of a human protein in bacteria by genetic engineering is possible because:
A)
bacterial cell can carry out the RNA splicing reactions
done
clear
B)
the human chromosome can replicate in bacterial cell
done
clear
C)
the mechanism of gene regulation is identical in humans and bacteria
done
clear
D)
the genetic code is universal
done
clear
View Answer play_arrow
-
question_answer132) From the following statements select the wrong one:
A)
millepedes have two pairs of appendages in each segment of the body
done
clear
B)
prawn has two pairs of antennae
done
clear
C)
animals belonging to phylum-Porifera are exclusively marine
done
clear
D)
nematocysts are characteristic of the phylum-Cnidaria
done
clear
View Answer play_arrow
-
question_answer133) Nucleotide are building blocks of nucleic acids, nucleotide is a composite molecule formed by:
A)
(base-sugar-phosphate)
done
clear
B)
base-sugar-OH
done
clear
C)
base-sugar-phosphate
done
clear
D)
sugar-phosphate
done
clear
View Answer play_arrow
-
question_answer134) More than 70% of world's freshwater is contained in:
A)
Antarctica
done
clear
B)
Glaciers and Mountains
done
clear
C)
Greenland
done
clear
D)
Polar ice
done
clear
View Answer play_arrow
-
question_answer135) Which one of the following pairs is mismatched?
A)
Biomass burning?release of \[C{{O}_{2}}\]
done
clear
B)
Fossil fuel burning?release of \[C{{O}_{2}}\]
done
clear
C)
Nuclear power?radioactive wastes
done
clear
D)
Solar energy?green house effect
done
clear
View Answer play_arrow
-
question_answer136) Which one of the following characters is not typical of the class-mammalia?
A)
Seven cervical vertebrae
done
clear
B)
Thecodont dentition
done
clear
C)
Ten pairs of cranial nerves
done
clear
D)
Alveolar lungs
done
clear
View Answer play_arrow
-
question_answer137) Which of the following is the simplest amino acid?
A)
Tyrosine
done
clear
B)
Asparagine
done
clear
C)
Glycine
done
clear
D)
Alanine
done
clear
View Answer play_arrow
-
question_answer138) Barophilic prokaryotes:
A)
grow slowly in highly alkaline frozen lakes at high altitudes
done
clear
B)
occur in water containing high concentrations of barium hydroxide
done
clear
C)
grow and multiply in very deep marine sediments
done
clear
D)
readily grown and divides in sea water enriched in any soluble salt of barium
done
clear
View Answer play_arrow
-
question_answer139) Auxospores and hormocysts are formed, respectively, by:
A)
several diatoms and a few cyanobacteria
done
clear
B)
several cyanobacteria and several diatoms
done
clear
C)
some diatoms and several cyanobacteria
done
clear
D)
some cyanobacteria and many diatoms
done
clear
View Answer play_arrow
-
question_answer140) Enzymes, vitamins and hormones can be classified into a single category of biological chemicals, because all of these:
A)
enhance oxidative metabolism
done
clear
B)
are conjugated proteins
done
clear
C)
are exclusively synthesized in the body of a living organism as at present
done
clear
D)
help in regulating metabolism
done
clear
View Answer play_arrow
-
question_answer141) Which one of the following phenomena supports Darwin's concept of natural selection in organic evolution?
A)
Development of transgenic animals
done
clear
B)
Production of 'Dolly? the sheep by cloning
done
clear
C)
Prevalence of pesticide resistant insects
done
clear
D)
Development of organs from 'stem cells' for organ transplantation
done
clear
View Answer play_arrow
-
question_answer142) As compared to a \[{{C}_{3}}\] plant, how many additional molecules of ATP are needed for net production of one molecule of hexose sugar by \[{{C}_{4}}\] plants:
A)
2
done
clear
B)
6
done
clear
C)
0
done
clear
D)
12
done
clear
View Answer play_arrow
-
question_answer143) In a man, abducens nerve is injured. Which one of the following functions will be affected?
A)
Movement of the eye ball
done
clear
B)
Swallowing
done
clear
C)
Movement of the tongue
done
clear
D)
Movement of the neck
done
clear
View Answer play_arrow
-
question_answer144) An important step in the manufacture of pulp for paper industry from the woody tissues of plants is the:
A)
preparation of pure cellulose by removing lignin
done
clear
B)
removal of oils present in the wood by treatment with suitable chemicals
done
clear
C)
removal of water from the wood by prolonged heating at approximately \[{{50}^{o}}C\]
done
clear
D)
treatment of wood with chemical that breakdown cellulose
done
clear
View Answer play_arrow
-
question_answer145) Protein synthesis in an animal cell occurs:
A)
only on the ribosomes present in cytosol
done
clear
B)
on ribosomes present in cytoplasm as well as in mitochondria
done
clear
C)
only on ribosomes attached to the nuclear envelope and endoplasmic reticulum
done
clear
D)
on ribosomes present in the nucleolus as well as in cytoplasm
done
clear
View Answer play_arrow
-
question_answer146) Which of the following is not true for a species?
A)
Members of a species can interbreed
done
clear
B)
Variations occurs among members of a species
done
clear
C)
Each species is reproductively isolated from every other species
done
clear
D)
Gene flow does not occur between the populations of a species
done
clear
View Answer play_arrow
-
question_answer147) One of the most important functions of botanical gardens is that:
A)
one can observe tropical plants there
done
clear
B)
they allow ex situ conservation of germplasm
done
clear
C)
they provide the natural habitat for wild life
done
clear
D)
they provide a beautiful area for recreation
done
clear
View Answer play_arrow
-
question_answer148) The ability of the venus flytrap to capture insects is due to:
A)
chemical stimulation by the prey
done
clear
B)
a passive process requiring no special ability on the part of the plant
done
clear
C)
specialized ?muscle-like? cells
done
clear
D)
rapid turgor pressure changes
done
clear
View Answer play_arrow
-
question_answer149) Animals have the innate ability to escape from predation. Examples for the same are given below. Select the incorrect example:
A)
enlargement of body size by swallowing air in puffer fish
done
clear
B)
melanism in moths
done
clear
C)
poison fangs in snakes
done
clear
D)
colour change in chameleon
done
clear
View Answer play_arrow
-
question_answer150) At a particular locus, frequency of 'A' allele is 0.6 and that of 'a' is 0.4. What would be the frequency of heterozygotes in a random mating population at equilibrium?
A)
0.16
done
clear
B)
0.48
done
clear
C)
0.36
done
clear
D)
0.24
done
clear
View Answer play_arrow
-
question_answer151) Which one of the following experiments suggests that simplest living organisms could not have originated spontaneously from non-living matter?
A)
Microbes did not appear in stored meat
done
clear
B)
Larvae could appear in decaying organic matter
done
clear
C)
Microbes appeared from unsterilized organic matter
done
clear
D)
Meat was not spoiled, when heated and kept sealed in a vessel
done
clear
View Answer play_arrow
-
question_answer152) Biodiversity act of India was passed by the Parliament in the year:
A)
1996
done
clear
B)
1992
done
clear
C)
2002
done
clear
D)
2000
done
clear
View Answer play_arrow
-
question_answer153) During which stage in the complete oxidation of glucose are the greatest number of ATP molecules formed from ADP?
A)
Conversion of pyruvic acid to acetyl Co-A
done
clear
B)
Electron transport chain
done
clear
C)
Glycolysis
done
clear
D)
Krebs cycle
done
clear
View Answer play_arrow
-
question_answer154) A woman with normal vision, but whose father was colour blind, marries a colourblind man. Suppose that the fourth child of this couple was a boy. This boy:
A)
must have normal colour vision
done
clear
B)
will be partially colourblind since he is heterozygous for the colourblind mutant allele
done
clear
C)
must be colourblind
done
clear
D)
may be colourblind or may be of normal vision
done
clear
View Answer play_arrow
-
question_answer155) Ectophloic siphonostele is found in:
A)
Adiantum and Cucurbitaceae
done
clear
B)
Osmunda and Equisetum
done
clear
C)
Marsilea and Botiychium
done
clear
D)
Dicksonia and maiden hair fern
done
clear
View Answer play_arrow
-
question_answer156) Which of the following represents the endible part of the fruit of litchi?
A)
Pericarp
done
clear
B)
Mesocarp
done
clear
C)
Juicy aril
done
clear
D)
Endocarp
done
clear
View Answer play_arrow
-
question_answer157) Carbohydrates, the most abundant biomoiecules on earth, are produced by:
A)
all bacteria, fungi and algae
done
clear
B)
fungi, algae and green plant cells
done
clear
C)
some bacteria, algae and green plant cells
done
clear
D)
viruses, fungi and bacteria
done
clear
View Answer play_arrow
-
question_answer158) Identify the correctly matched pair:
A)
Montreal protocol - Global warming
done
clear
B)
Kyoto protocol - Climatic change
done
clear
C)
Ramsar convention - Ground water pollution
done
clear
D)
Basal convention - Biodiversity conservation
done
clear
View Answer play_arrow
-
question_answer159) Which of the following is not a hereditary disease?
A)
Cretinism
done
clear
B)
Cystic fibrosis
done
clear
C)
Thalassaemia
done
clear
D)
Haemophilia
done
clear
View Answer play_arrow
-
question_answer160) The deficiencies of micro-nutrients, not only affects growth of plants but also vital functions such as photosynthetic and mitochondrial electron flow. Among the list given below which group of three elements shall affect most, both photosynthetic and mitochondrial electron transport?
A)
Cu, Mn, Fe
done
clear
B)
Co, Ni, Mo
done
clear
C)
Mn, Co, Ca
done
clear
D)
Ca, K, Na
done
clear
View Answer play_arrow
-
question_answer161) de Vries gave his mutation theory on organic evolution while working on:
A)
Akhea rosea
done
clear
B)
Drosophila melanogaster
done
clear
C)
Oenothera lamarckiana
done
clear
D)
Pisum sativum
done
clear
View Answer play_arrow
-
question_answer162) One of the examples of the action of the autonomous nervous system is:
A)
knee-jerk response
done
clear
B)
pupillary reflex
done
clear
C)
swallowing of food
done
clear
D)
peristalsis of the intestines
done
clear
View Answer play_arrow
-
question_answer163) Which of the following is not used for disinfection of drinking water?
A)
Phenyl
done
clear
B)
Chloramine
done
clear
C)
Chlorine
done
clear
D)
Ozone
done
clear
View Answer play_arrow
-
question_answer164) Chemiosmotic theory of ATP synthesis in the chloroplasts and mitochondria is based on:
A)
proton gradient
done
clear
B)
accumulation of K ions
done
clear
C)
accumulation of Na ions
done
clear
D)
membrane potential
done
clear
View Answer play_arrow
-
question_answer165) Parkinson's disease (characterized by tremors and progressive rigidity of limbs) is caused by degeneration of brain neurons that are involved in movement control and make use of neurotransmitter:
A)
acetylcholine
done
clear
B)
norepinephrine
done
clear
C)
dopamine
done
clear
D)
GABA
done
clear
View Answer play_arrow
-
question_answer166) All of the following statements concerning the actinomycetous filamentous soil bacterium Frankia are correct except that Frankia:
A)
can induce root nodules on many plant species
done
clear
B)
can fix nitrogen in the free-living state
done
clear
C)
like Rhizobium, it usually infects its host plant through root hair deformation and stimulates cell proliferation in the host's cortex
done
clear
D)
forms specialized vesicles in which the nitrogenase is protected from oxygen by a chemical barrier involving triterpene hopanoids
done
clear
View Answer play_arrow
-
question_answer167) An acromian process is characteristically found in die:
A)
pelvic girdle of mammals
done
clear
B)
skull of frog
done
clear
C)
pectoral girdle of mammals
done
clear
D)
sperm of mammals
done
clear
View Answer play_arrow
-
question_answer168) In a type of apomixis known as adventive embryony, embryos develop directly from the:
A)
nucellus or integuments
done
clear
B)
synergids or antipodals in an embryo sac
done
clear
C)
accessory embryo sacs in the ovule
done
clear
D)
zygote
done
clear
View Answer play_arrow
-
question_answer169) Through which cell of the embryo sac, does the pollen tube enter the embryo sac?
A)
Egg cell
done
clear
B)
Central cell
done
clear
C)
Persistant synergid
done
clear
D)
Degenerated synergid
done
clear
View Answer play_arrow
-
question_answer170) Epithelial cells of the intestine involved in food absorption have on their surface:
A)
pinocytic vesicles
done
clear
B)
phagocytic vesicles
done
clear
C)
zymogen granules
done
clear
D)
micro-villi
done
clear
View Answer play_arrow
-
question_answer171) A patient is generally advised to specially; consume more meat, lentils, milk and eggs in diet only when he suffers from:
A)
kwashiorkor
done
clear
B)
rickets
done
clear
C)
anaemia
done
clear
D)
scurvy
done
clear
View Answer play_arrow
-
question_answer172) Which one of the following pairs is mismatched?
A)
Savanna ? Acacia trees
done
clear
B)
Prairie ? epiphytes
done
clear
C)
Tundra ? permafrost
done
clear
D)
Coniferous forest ? evergreen trees
done
clear
View Answer play_arrow
-
question_answer173) Which of the following is the relatively most accurate method for dating of fossils?
A)
Potassium - argon method
done
clear
B)
Uranium- lead method
done
clear
C)
Electron - spin resonance method
done
clear
D)
Radio - carbon method
done
clear
View Answer play_arrow
-
question_answer174) Which one of the following represents an ovule, where the embryo sac becomes horse-shoe shaped and the funiculus and micropyle are close to each other?
A)
Circinotropous
done
clear
B)
Anatropous
done
clear
C)
Amphitropous
done
clear
D)
Atropous
done
clear
View Answer play_arrow
-
question_answer175) Potometer works on the principle of:
A)
amount of water absorbed equals the amount transpired
done
clear
B)
osmotic pressure
done
clear
C)
root pressure
done
clear
D)
potential difference between the tip of the tube and that of the plant
done
clear
View Answer play_arrow
-
question_answer176) In a woody dicotyledonous tree, which of the following parts will mainly consist of primary tissues?
A)
Stem and root
done
clear
B)
All parts
done
clear
C)
Shoot tips and root tips
done
clear
D)
Flowers, fruits and leaves
done
clear
View Answer play_arrow
-
question_answer177) Which one of the following makes use of RNA as a template to synthesize DNA?
A)
Reverse transcriptase
done
clear
B)
DNA dependant RNA polymerase
done
clear
C)
DNA polymerase
done
clear
D)
RNA polymerase
done
clear
View Answer play_arrow
-
question_answer178) In contrast to annelids the platyhelminths show:
A)
radial symmetry
done
clear
B)
presence of pseudocoel
done
clear
C)
bilateral symmetry
done
clear
D)
absence of body cavity
done
clear
View Answer play_arrow
-
question_answer179) Which of the following statements regarding enzyme inhibition is correct?
A)
Non-competitive inhibition of an enzyme can he overcome by adding large amount of substrate
done
clear
B)
Competitive inhibition is seen when a substrate competes with an enzyme for binding to an inhibitor protein
done
clear
C)
Competitive inhibition is seen when the substrate and the inhibitor compete
done
clear
D)
Non-competitive inhibitors often bind to the enzyme irreversibly
done
clear
View Answer play_arrow
-
question_answer180) Which of the following pairs is correctly matched?
A)
Cartilaginous joint ? skull bones
done
clear
B)
Hinge joint ? between vertebrae
done
clear
C)
Fibrous joint ? between phalanges
done
clear
D)
Gliding joint ? between zygapophyses of the successive vertebrae
done
clear
View Answer play_arrow
-
question_answer181) The catalytic efficiency of two different enzymes can be compared by the:
A)
the Revalue
done
clear
B)
the pH optimum value
done
clear
C)
formation of the product
done
clear
D)
molecular size of the enzyme
done
clear
View Answer play_arrow
-
question_answer182) Using imprints from a plate with complete medium and carrying bacterial colonies, you can select streptomycin resistant mutants and prove that such mutations do not originate as adaptation. These imprints need to be used:
A)
only on plates with streptomycin
done
clear
B)
on plates with minimal medium
done
clear
C)
only on plates without streptomycin
done
clear
D)
on plates with and without streptomycin
done
clear
View Answer play_arrow
-
question_answer183) Which of the following is generally used for induced mutagenesis in crop plants?
A)
Alpha particles
done
clear
B)
X-rays
done
clear
C)
UV (260 nm)
done
clear
D)
Gamma rays (from cobalt 60)
done
clear
View Answer play_arrow
-
question_answer184) Haemophilia is more commonly seen in human males than in human females because:
A)
this disease is due to an X-linked dominant mutation
done
clear
B)
a greater proportion of girls die in infancy
done
clear
C)
this disease is due to an X-linked recessive mutation
done
clear
D)
this disease is due to a Y-linked recessive mutation
done
clear
View Answer play_arrow
-
question_answer185) A woman with 47 chromosomes due to three copies of chromosome 21 is characterized by:
A)
Down syndrome
done
clear
B)
triploidy
done
clear
C)
Turner syndrome
done
clear
D)
super femaleness
done
clear
View Answer play_arrow
-
question_answer186) In order to find out the different types of gametes produced by a pea plant having the genotype AaBb, it should be crossed to a plant with the genotype:
A)
aaBB
done
clear
B)
AaBb
done
clear
C)
AABB
done
clear
D)
aabb
done
clear
View Answer play_arrow
-
question_answer187) Four healthy people in their twenties got involved in injuries resulting in damage and death of a few cells of the following. Which of the cells are least likely to be replaced by new cells?
A)
Osteocytes
done
clear
B)
Malpighian layer of the skin
done
clear
C)
Liver cells
done
clear
D)
Neurons
done
clear
View Answer play_arrow
-
question_answer188) Secretin and cholecystokinin are digestive hormones. They are secreted in:
A)
oesophagus
done
clear
B)
ileum
done
clear
C)
duodenum
done
clear
D)
pyloric stomach
done
clear
View Answer play_arrow
-
question_answer189) Which of the following unicellular organism has a macro-nucleus for trophic function and one or more micro-nuclei for reproduction?
A)
Euglena
done
clear
B)
Amoeba
done
clear
C)
Paramecium
done
clear
D)
Trypanosoma
done
clear
View Answer play_arrow
-
question_answer190) AIDS is caused by HTV that principally infects:
A)
all lymphocytes
done
clear
B)
activator B cells
done
clear
C)
\[{{T}_{4}}\] lymphocytes
done
clear
D)
cytotoxic T cells
done
clear
View Answer play_arrow
-
question_answer191) According to widely accepted ?fluid mosaic model? cell membranes are semi-fluid, where lipids and integral proteins can diffuse randomly. In recent years, this model has been modified in several respects. In this regard, which of the following statements is incorrect?
A)
Proteins in cell membranes can travel within the lipid bilayer
done
clear
B)
Proteins can remain confined within certain domains of the membrane
done
clear
C)
Proteins can also undergo flip-flop movements in the lipid bilayer
done
clear
D)
Many proteins remain completely embedded within the lipid bilayer
done
clear
View Answer play_arrow
-
question_answer192) If mammalian ovum fails 10 get fertilized, which one of the following is unlikely?
A)
Corpus luteum will disintegrate
done
clear
B)
Estrogen secretion further decreases
done
clear
C)
Primary follicle starts developing
done
clear
D)
Progesterone secretion rapidly declines
done
clear
View Answer play_arrow
-
question_answer193) A person is undergoing prolonged fasting. His urine will be found to contain abnormal quantities of:
A)
fats
done
clear
B)
ketones
done
clear
C)
amino acids
done
clear
D)
glucose
done
clear
View Answer play_arrow
-
question_answer194) Why is vivipary an undesirable character for annual crop plants?
A)
It reduces the vigour of plant
done
clear
B)
The seeds cannot be stored under normal conditions for the next season
done
clear
C)
The seeds exhibit long dormancy
done
clear
D)
It adversely affects the fertility of the plant
done
clear
View Answer play_arrow
-
question_answer195) Bacillus thuringiensis (Bt) strains have been used for designing novel:
A)
bio-metallurgical technique
done
clear
B)
bio-mineralization processes
done
clear
C)
bio-insecticidal plants
done
clear
D)
bio-fertilizers
done
clear
View Answer play_arrow
-
question_answer196) The salivary gland chromosomes in the dipteran larvae, are useful in gene mapping because:
A)
these are much longer in size
done
clear
B)
these are easy to stain
done
clear
C)
these are fused
done
clear
D)
they have endoreduplicated chromosomes
done
clear
View Answer play_arrow
-
question_answer197) Which group of three of the following five statements (1-5) contain is all three correct statements regarding beri-beri? A. A crippling disease prevalent among the native population of sub-Sahara Africa. B. A deficiency disease caused by lack of thiamine (vitamin \[{{B}_{1}}\]). C.A nutritional disorder in infants and young children when the diet is persistently deficient in essential protein. D. Occurs in those countries where the staple diet is polished rice. E. The symptoms are pain from neuritis, paralysis, muscle wasting, progressive oedema, mental deterioration and finally heart failure.
A)
A, B and D
done
clear
B)
B, C and E
done
clear
C)
A, C and E
done
clear
D)
B, D and E
done
clear
View Answer play_arrow
-
question_answer198) Photosynthetic Active Radiation (PAR) has the following range of wavelengths:
A)
400-700 nm
done
clear
B)
450-950 nm
done
clear
C)
340-450-nm
done
clear
D)
500-600 nm
done
clear
View Answer play_arrow
-
question_answer199) Golden rice is a transgenic crop of the future with the following improved trait:
A)
high lysine (essential amino acid) content
done
clear
B)
insect resistance
done
clear
C)
high protein content
done
clear
D)
high vitamin A content
done
clear
View Answer play_arrow
-
question_answer200) Which one of the following depresses brain activity and produces feelings of calmness, relaxation and drowsiness?
A)
Valium
done
clear
B)
Morphine
done
clear
C)
Hashish
done
clear
D)
Amphetamines
done
clear
View Answer play_arrow
 15 V
15 V  \[\frac{22}{3}\]
\[\frac{22}{3}\] 





 The structure of die product D would be:
The structure of die product D would be:
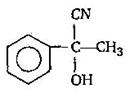
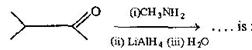
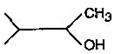
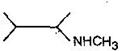
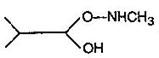
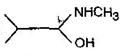
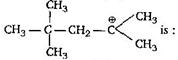
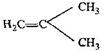
 is:
is:
