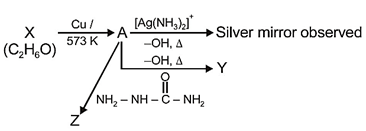
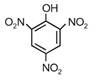
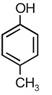
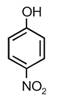
question_answer 1) A spring of force constant k is cut into lengths of ratio 1 : 2 : 3. They are connected in series and the new force constant is k?. Then they are connected in parallel and force constant is k?. Then k? : k?? is
A)
1 : 14
done
clear
B)
1 : 6
done
clear
C)
1 : 9
done
clear
D)
1 : 11
done
clear
View Answer play_arrow
question_answer 2)
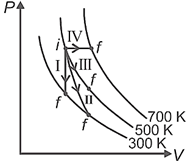
Thermodynamic processes are indicated in the following diagram.
Column-I
Column-II
P. Process I
a. Adiabatic
Q. Process II
b. Isobaric
R. Process III
c. Isochoric
S. Process IV
d. Isothermal
A)
\[P\to d,\,Q\to b,\,R\to a,\,S\to c\]
done
clear
B)
\[P\to a,\,Q\to c,\,R\to d,\,S\to b\]
done
clear
C)
\[P\to c,\,Q\to a,\,R\to d,\,S\to b\]
done
clear
D)
\[P\to c,\,Q\to d,\,R\to b,\,S\to a\]
done
clear
View Answer play_arrow
question_answer 3) A capacitor is charged by a battery. The battery is removed and another identical uncharged capacitor is connected in parallel. The total electrostatic energy of resulting system
A)
Increases by a factor of 2
done
clear
B)
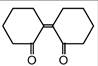
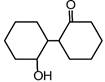
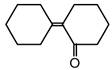
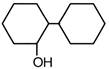
Increases by a factor of 4
done
clear
C)
Decreases by a factor of 2
done
clear
D)
Remains the same
done
clear
View Answer play_arrow
question_answer 4)
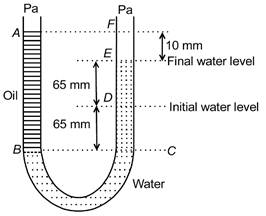
A U tube with both ends open to the atmosphere, is partially filled with water. Oil, which is immiscible with water, is poured into one side until it stands at a distance of 10 mm above the water level on the other side. Meanwhile the water rises by 65 mm from its original level (see diagram). The density of the oil is
A)
\[928\,kg\,{{m}^{-3}}\]
done
clear
B)
\[650\,kg\,{{m}^{-3}}\]
done
clear
C)
\[425\,kg\,{{m}^{-3}}\]
done
clear
D)
\[800\,kg\,{{m}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 5) The de-Broglie wavelength of a neutron in thermal equilibrium with heavy water at a temperature T (Kelvin) and mass m, is
A)
\[\frac{2h}{\sqrt{mkT}}\]
done
clear
B)
\[\frac{h}{\sqrt{mkT}}\]
done
clear
C)
\[\frac{h}{\sqrt{3mkT}}\]
done
clear
D)
\[\frac{2h}{\sqrt{3mkT}}\]
done
clear
View Answer play_arrow
question_answer 6) The acceleration due to gravity at a height 1 km above the earth is the same as at a depth d below the surface of earth. Then
A)
\[d=2\,km\]
done
clear
B)
\[d=\frac{1}{2}\,km\]
done
clear
C)
\[d=\,1\,km\]
done
clear
D)
\[d=\frac{\begin{align} & 3 \\ & 3 \\ \end{align}}{2}\,km\]
done
clear
View Answer play_arrow
question_answer 7) The\[x\]and y coordinates of the particle at any time are \[x=5t-2{{t}^{2}}\]and \[y=10t\]respectively, where\[x\] and y are in meters and t in seconds. The acceleration of the particle at t = 2 s is
A)
\[-8\,m/{{s}^{2}}\]
done
clear
B)
0
done
clear
C)
\[5\,m/{{s}^{2}}\]
done
clear
D)
\[-4\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 8) In a common emitter transistor amplifier the audio signal voltage across the collector is 3 V. The resistance of collector is\[3\,k\Omega .\]If current gain is 100 and the base resistance is \[2\,k\Omega ,\] the voltage and power gain of the amplifier is
A)
20 and 2000
done
clear
B)
200 and 1000
done
clear
C)
15 and 200
done
clear
D)
150 and 15000
done
clear
View Answer play_arrow
question_answer 9)
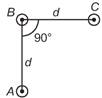
An arrangement of three parallel straight wires placed perpendicular to plane of paper carrying same current ?I? along the same direction is shown in Fig. Magnitude of force per unit length on the middle wire 'B' is given by
A)
\[\frac{{{\mu }_{0}}{{l}^{2}}}{\sqrt{2}\pi d}\]
done
clear
B)
\[\frac{{{\mu }_{0}}{{l}^{2}}}{2\pi d}\]
done
clear
C)
\[\frac{2{{\mu }_{0}}{{l}^{2}}}{\pi d}\]
done
clear
D)
\[\frac{\sqrt{2}{{\mu }_{0}}{{l}^{2}}}{\pi d}\]
done
clear
View Answer play_arrow
question_answer 10) Two astronauts are floating in gravitational free space after having lost contact with their spaceship. The two will:
A)
Will become stationary
done
clear
B)
Keep floating at the same distance between them
done
clear
C)
Move towards each other
done
clear
D)
Move away from each other
done
clear
View Answer play_arrow
question_answer 11) A Carnot engine having an efficiency of\[\frac{1}{10}\] as heat engine, is used as a refrigerator. If the work done on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is
A)
100 J
done
clear
B)
1 J
done
clear
C)
90 J
done
clear
D)
99 J
done
clear
View Answer play_arrow
question_answer 12) A 250-Turn rectangular coil of length 2.1 cm and width 1.25 cm carries a current of \[85\,\mu A\]and subjected to a magnetic field of strength 0.85 T. Work done for rotating the coil by \[{{180}^{o}}\] against the torque is
A)
\[1.15\,\mu J\]
done
clear
B)
\[9.1\,\mu J\]
done
clear
C)
\[4.55\,\mu J\]
done
clear
D)
\[2.3\,\mu J\]
done
clear
View Answer play_arrow
question_answer 13) Which one of the following represents forward bias diode?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 14) The ratio of wavelengths of the last line of Balmer series and the last line of Lyman series is
A)
0.5
done
clear
B)
2
done
clear
C)
1
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 15)
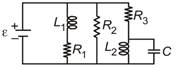
Figure shows a circuit contains three identical resistors with resistance\[R=9.0\,\Omega \]each, two identical inductors with inductance L = 2.0 mH each, and an ideal battery with emf \[\varepsilon =18\,V.\] The current 'i' through the battery just after the switch closed is
A)
0 ampere
done
clear
B)
2 mA
done
clear
C)
0.2 A
done
clear
D)
2 A
done
clear
View Answer play_arrow
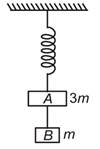
question_answer 16)
Two blocks A and B of masses 3m and m respectively are connected by a massless and inextensible string. The whole system is suspended by a massless spring as shown in figure. The magnitudes of acceleration of A and B immediately after the string is cut, are respectively
A)
\[\frac{g}{3},\frac{g}{3}\]
done
clear
B)
\[g,\frac{g}{3}\]
done
clear
C)
\[\frac{g}{3},g\]
done
clear
D)
\[g,g\]
done
clear
View Answer play_arrow
question_answer 17) A long solenoid of diameter 0.1 m has\[2\times {{10}^{4}}\] turns per meter. At the centre of the solenoid, a coil of 100 turns and radius 0.01 m is placed with its axis coinciding with the solenoid axis. The current in the solenoid reduces at a constant rate to 0 A from 4 A in 0.05 s. If the resistance of the coil is\[10{{\pi }^{2}}\Omega ,\] the total charge flowing through the coil during this time is
A)
\[16\pi \,\mu C\]
done
clear
B)
\[32\pi \,\mu C\]
done
clear
C)
\[16\mu C\]
done
clear
D)
\[32\,\mu C\]
done
clear
View Answer play_arrow
question_answer 18) A physical quantity of the dimensions of length that can be formed out of c, G and\[\frac{{{e}^{2}}}{4\pi {{\varepsilon }_{0}}}\] is [c is velocity of light, G is universal constant of gravitation and e is charge]
A)
\[\frac{1}{c}G\frac{{{e}^{2}}}{4\pi {{\varepsilon }_{0}}}\]
done
clear
B)
\[\frac{1}{{{c}^{2}}}{{\left[ G\frac{{{e}^{2}}}{4\pi {{\varepsilon }_{0}}} \right]}^{\frac{1}{2}}}\]
done
clear
C)
\[{{c}^{2}}{{\left[ G\frac{{{e}^{2}}}{4\pi {{\varepsilon }_{0}}} \right]}^{\frac{1}{2}}}\]
done
clear
D)
\[\frac{1}{{{c}^{2}}}{{\left[ \frac{{{e}^{2}}}{G4\pi {{\varepsilon }_{0}}} \right]}^{\frac{1}{2}}}\]
done
clear
View Answer play_arrow
question_answer 19) In an electromagnetic wave in free space the root mean square value of the electric field is \[{{E}_{rms}}=6\,V/m.\]The peak value of the magnetic field is
A)
\[4.23\times {{10}^{-8}}T\]
done
clear
B)
\[1.41\times {{10}^{-8}}T\]
done
clear
C)
\[2.83\times {{10}^{-8}}T\]
done
clear
D)
\[0.70\times {{10}^{-8}}T\]
done
clear
View Answer play_arrow
question_answer 20) The resistance of a wire is 'R' ohm. If it is melted and stretched to 'n' times its original length, its new resistance will be
A)
\[\frac{R}{{{n}^{2}}}\]
done
clear
B)
\[nR\]
done
clear
C)
\[\frac{R}{n}\]
done
clear
D)
\[{{n}^{2}}R\]
done
clear
View Answer play_arrow
question_answer 21) The ratio of resolving powers of an optical microscope for two wavelengths \[{{\lambda }_{1}}=4000\,\overset{\text{o}}{\mathop{\text{A}}}\,\]and \[{{\lambda }_{2}}=6000\,\overset{\text{o}}{\mathop{\text{A}}}\,\]is
A)
16 : 81
done
clear
B)
8 : 27
done
clear
C)
9 : 4
done
clear
D)
3 : 2
done
clear
View Answer play_arrow
question_answer 22) A thin prism having refracting angle\[{{10}^{o}}\] is made of glass of refractive index 1.42. This prism is combined with another thin prism of glass of refractive index 1.7. This combination produces dispersion without deviation. The refracting angle of second prism should be
A)
\[{{10}^{o}}\]
done
clear
B)
\[{{4}^{o}}\]
done
clear
C)
\[{{6}^{o}}\]
done
clear
D)
\[{{8}^{o}}\]
done
clear
View Answer play_arrow
question_answer 23) Two Polaroids \[{{P}_{1}}\]and\[{{P}_{2}}\]are placed with their axis perpendicular to each other. Un polarised light \[{{l}_{0}}\]is incident on\[{{P}_{1}}.\]A third polaroid\[{{P}_{3}}\]is kept in between\[{{P}_{1}}\]and\[{{P}_{2}}\]such that its axis makes an angle\[{{45}^{o}}\]with that of\[{{P}_{1}}.\]The intensity of transmitted light through\[{{P}_{2}}\] is
A)
\[\frac{{{l}_{0}}}{16}\]
done
clear
B)
\[\frac{{{l}_{0}}}{2}\]
done
clear
C)
\[\frac{{{l}_{0}}}{4}\]
done
clear
D)
\[\frac{{{l}_{0}}}{8}\]
done
clear
View Answer play_arrow
question_answer 24) A potentiometer is an accurate and versatile device to make electrical measurements of E.M.F, because the method involves:
A)
A combination of cells, galvanometer and resistances
done
clear
B)
Cells
done
clear
C)
Potential gradients
done
clear
D)
A condition of no current flow through the galvanometer
done
clear
View Answer play_arrow
question_answer 25) The two nearest harmonics of a tube closed at one end and open at other end are 220 Hz and 260 Hz. What is the fundamental frequency of the system?
A)
40 Hz
done
clear
B)
10 Hz
done
clear
C)
20 Hz
done
clear
D)
30 Hz
done
clear
View Answer play_arrow
question_answer 26) A beam of light from a source L is incident normally on a plane mirror fixed at a certain distance x from the source. The beam is reflected back as a spot on a scale placed just above the source L. When the mirror is rotated through a small angle\[\theta ,\]the spot of the light is found to move through a distance y on the scale. The angle\[\theta \] is given by
A)
\[\frac{x}{y}\]
done
clear
B)
\[\frac{y}{2x}\]
done
clear
C)
\[\frac{y}{x}\]
done
clear
D)
\[\frac{x}{2y}\]
done
clear
View Answer play_arrow
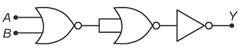
question_answer 27)
The given electrical network is equivalent to
A)
NOT gate
done
clear
B)
AND gate
done
clear
C)
OR gate
done
clear
D)
NOR gate
done
clear
View Answer play_arrow
question_answer 28) A particle executes linear simple harmonic motion with an amplitude of 3 cm. When the particle is at 2 cm from the mean position, the magnitude of its velocity is equal to that of its acceleration. Then its time period in seconds is
A)
\[\frac{2\pi }{\sqrt{3}}\]
done
clear
B)
\[\frac{\sqrt{5}}{\pi }\]
done
clear
C)
\[\frac{\sqrt{5}}{2\pi }\]
done
clear
D)
\[\frac{4\pi }{\sqrt{5}}\]
done
clear
View Answer play_arrow
question_answer 29) Preeti reached the metro station and found that the escalator was not working. She walked up the stationary escalator in time\[{{t}_{1}}.\]On other days, if she remains stationary on the moving escalator, then the escalator takes her up in time\[{{t}_{2}}.\]The time taken by her to walk up on the moving escalator will be
A)
\[{{t}_{1}}-{{t}_{2}}\]
done
clear
B)
\[\frac{{{t}_{1}}+{{t}_{2}}}{2}\]
done
clear
C)
\[\frac{{{t}_{1}}{{t}_{2}}}{{{t}_{2}}-{{t}_{1}}}\]
done
clear
D)
\[\frac{{{t}_{1}}{{t}_{2}}}{{{t}_{2}}+{{t}_{1}}}\]
done
clear
View Answer play_arrow
question_answer 30) Two discs of same moment of inertia rotating about their regular axis passing through centre and perpendicular to the plane of disc with angular velocities \[{{\omega }_{1}}\] and\[{{\omega }_{2}}.\]They are brought into contact face to face coinciding the axis of rotation. The expression for loss of energy during this process is
A)
\[\frac{l}{8}{{({{\omega }_{1}}-{{\omega }_{2}})}^{2}}\]
done
clear
B)
\[\frac{1}{2}l{{({{\omega }_{1}}+{{\omega }_{2}})}^{2}}\]
done
clear
C)
\[\frac{1}{4}l{{({{\omega }_{1}}-{{\omega }_{2}})}^{2}}\]
done
clear
D)
\[l{{({{\omega }_{1}}-{{\omega }_{2}})}^{2}}\]
done
clear
View Answer play_arrow
question_answer 31) A gas mixture consists of 2 moles of\[{{O}_{2}}\]and 4 moles of Ar at temperature T. Neglecting all vibrational modes, the total internal energy of the system is
A)
\[11\,RT\]
done
clear
B)
\[4RT\]
done
clear
C)
\[15\,RT\]
done
clear
D)
\[9\,RT\]
done
clear
View Answer play_arrow
question_answer 32) The bulk modulus of a spherical object is 'B'. If it is subjected to uniform pressure 'p' , the fractional decrease in radius is
A)
\[\frac{\rho }{3B}\]
done
clear
B)
\[\frac{\rho }{B}\]
done
clear
C)
\[\frac{B}{3p}\]
done
clear
D)
\[\frac{3p}{B}\]
done
clear
View Answer play_arrow
question_answer 33) One end of string of length l is connected to a particle of mass 'm' and the other end is connected to a small peg on a smooth horizontal table. If the particle moves in circle with speed 'v', the net force on the particle (directed towards center) will be (T represents the tension in the string)
A)
Zero
done
clear
B)
T
done
clear
C)
\[T+\frac{m{{v}^{2}}}{l}\]
done
clear
D)
\[T-\frac{m{{v}^{2}}}{l}\]
done
clear
View Answer play_arrow
question_answer 34) A rope is wound around a hollow cylinder of mass 3 kg and radius 40 cm. What is the angular acceleration of the cylinder if the rope is pulled with a force of 30 N?
A)
\[~5\text{ }m/{{s}^{2}}\]
done
clear
B)
\[~25\text{ }m/{{s}^{2}}\]
done
clear
C)
\[~0.25\text{ }rad/{{s}^{2}}\]
done
clear
D)
\[~25\text{ }rad/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 35) Young?s double slit experiment is first performed in air and then in a medium other than air. It is found that 8th bright fringe in the medium lies where 5th dark fringe lies in air. The refractive index of the medium is nearly
A)
1.78
done
clear
B)
1.25
done
clear
C)
1.59
done
clear
D)
1.69
done
clear
View Answer play_arrow
question_answer 36) Suppose the charge of a proton and an electron differ slightly. One of them is ?e, the other is\[(e+\Delta e).\] If the net of electrostatic force and gravitational force between two hydrogen atoms placed at a distance d (much greater than atomic size) apart is zero, then \[\Delta e\] is of the order of [Given mass of hydrogen \[{{m}_{h}}=1.67\times {{10}^{-27}}kg]\]
A)
\[{{10}^{-47}}C\]
done
clear
B)
\[{{10}^{-20}}C\]
done
clear
C)
\[{{10}^{-23}}C\]
done
clear
D)
\[{{10}^{-37}}C\]
done
clear
View Answer play_arrow
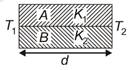
question_answer 37)
Two rods A and B of different materials are welded together as shown in figure. Their thermal conductivities are \[{{K}_{1}}\]and \[{{K}_{2}}.\]The thermal conductivity of the composite rod will be
A)
\[2({{K}_{1}}+{{K}_{2}})\]
done
clear
B)
\[\frac{{{K}_{1}}+{{K}_{2}}}{2}\]
done
clear
C)
\[\frac{3({{K}_{1}}+{{K}_{2}})}{2}\]
done
clear
D)
\[{{K}_{1}}+{{K}_{2}}\]
done
clear
View Answer play_arrow
question_answer 38) The photoelectric threshold wavelength of silver is\[3250\times {{10}^{-10}}m.\]The velocity of the electron ejected from a silver surface by ultraviolet light of wavelength \[2536\times {{10}^{-10}}m\]is (Given \[h=4.14\times {{10}^{-15}}eVs\]and\[c=3\times {{180}^{8}}\,m{{s}^{-1}}\])
A)
\[\approx 0.3\times {{10}^{6}}\,m{{s}^{-1}}\]
done
clear
B)
\[\approx 6\times {{10}^{5}}\,m{{s}^{-1}}\]
done
clear
C)
\[\approx 0.6\times {{10}^{6}}\,m{{s}^{-1}}\]
done
clear
D)
\[\approx 61\times {{10}^{3}}\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 39) Two cars moving in opposite directions approach each other with speed of 22 m/s and 16.5 m/s respectively. The driver of the first car blows a horn having a frequency 400 Hz. The frequency heard by the driver of the second car is [velocity of sound 340 m/s]
A)
448 Hz
done
clear
B)
350 Hz
done
clear
C)
361 Hz
done
clear
D)
411 Hz
done
clear
View Answer play_arrow
question_answer 40) Consider a drop of rain water having mass 1 g falling from a height of 1 km. It hits the ground with a speed of 50 m/s. Take g constant with a value\[10\,m/{{s}^{2}}.\]The work done by the (i) gravitational force and the (ii) resistive force of air is
A)
(i) 10 J (ii) -8.75 J
done
clear
B)
(i) -10 J (ii) -8.25 J
done
clear
C)
(i) 1.25 J (ii) -8.25 J
done
clear
D)
(i) 100 J (ii) 8.75 J
done
clear
View Answer play_arrow
question_answer 41) A spherical black body with a radius of 12 cm radiates 450 watt power at 500 K. If the radius were halved and the temperature doubled, the power radiated in watt would be
A)
1800
done
clear
B)
225
done
clear
C)
450
done
clear
D)
1000
done
clear
View Answer play_arrow
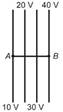
question_answer 42)
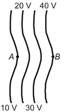
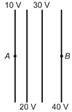
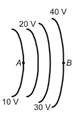
The diagrams below show regions of equal potentials. A positive charge is moved from A to B in each diagram.
A)
Maximum work is required to move q in figure [b].
done
clear
B)
Maximum work is required to move q in figure [c].
done
clear
C)
In all the four cases the work done is the same.
done
clear
D)
Minimum work is required to move q in figure [a].
done
clear
View Answer play_arrow
question_answer 43)
Which of the following statements are correct?
(A) Centre of mass of a body always coincides with the centre of gravity of the body.
(B) Centre of mass of a body is the point at which the total gravitational torque on the body is zero
(C) A couple on a body produce both translational and rotational motion in a body.
(D) Mechanical advantage greater than one means that small effort can be used to lift a large load.
A)
C and D
done
clear
B)
B and D
done
clear
C)
A and B
done
clear
D)
B and C
done
clear
View Answer play_arrow
question_answer 44) If\[{{\theta }_{1}}\]and\[{{\theta }_{2}}\]be the apparent angles of dip observed in two vertical planes at right angles to each other, then the true angle of dip\[\theta \] is given by
A)
\[{{\tan }^{2}}\theta ={{\tan }^{2}}{{\theta }_{1}}-{{\tan }^{2}}{{\theta }_{2}}\]
done
clear
B)
\[{{\cot }^{2}}\theta ={{\cot }^{2}}{{\theta }_{1}}+{{\cot }^{2}}{{\theta }_{2}}\]
done
clear
C)
\[{{\tan }^{2}}\theta ={{\tan }^{2}}{{\theta }_{1}}+{{\tan }^{2}}{{\theta }_{2}}\]
done
clear
D)
\[{{\cot }^{2}}\theta ={{\cot }^{2}}{{\theta }_{1}}-{{\cot }^{2}}{{\theta }_{2}}\]
done
clear
View Answer play_arrow
question_answer 45) Radioactive material 'A' has decay constant \['8\lambda '\]and material 'B' has decay constant \['\lambda '.\] Initially they have same number of nuclei. After what time, the ratio of number of nuclei of material 'B' to that 'A' will be \[\frac{1}{e}\]?
A)
\[\frac{1}{9\lambda }\]
done
clear
B)
\[\frac{1}{\lambda }\]
done
clear
C)
\[\frac{1}{7\lambda }\]
done
clear
D)
\[\frac{1}{8\lambda }\]
done
clear
View Answer play_arrow
question_answer 46) The equilibrium constants of the following are. \[{{N}_{2}}+3{{H}_{2}}\rightleftharpoons 2N{{H}_{3}}\,{{K}_{4}}\] \[{{N}_{2}}+{{O}_{2}}\rightleftharpoons 2NO\,{{K}_{2}}\] \[{{H}_{2}}+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}{{H}_{2}}O\,{{K}_{3}}\] The equilibrium constant (K) of the reaction \[2N{{H}_{3}}+\frac{5}{2}{{O}_{2}}\xrightarrow{k}2NO+3{{H}_{2}}O,\]will be
A)
\[K_{2}^{3}{{K}_{3}}/{{K}_{1}}\]
done
clear
B)
\[{{K}_{1}}K_{3}^{3}/{{K}_{2}}\]
done
clear
C)
\[{{K}_{2}}K_{3}^{3}/{{K}_{1}}\]
done
clear
D)
\[{{K}_{2}}{{K}_{3}}/{{K}_{1}}\]
done
clear
View Answer play_arrow
question_answer 47) The heating of phenyl-methyl ethers with HI produces.
A)
Benzene
done
clear
B)
Ethyl chlorides
done
clear
C)
Iodobenzene
done
clear
D)
Phenol
done
clear
View Answer play_arrow
question_answer 48) The most suitable method of separation of 1 : 1 mixture of ortho and para-nitrophenols is
A)
Steam distillation
done
clear
B)
Sublimation
done
clear
C)
Chromatography
done
clear
D)
Crystallisation
done
clear
View Answer play_arrow
question_answer 49) Predict the correct intermediate and product in the following reaction \[{{H}_{3}}C-C\equiv CH\xrightarrow[HgS{{O}_{4}}]{{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}}\underset{\text{(A)}}{\mathop{\text{intermediate}}}\,\xrightarrow{{}}\underset{\text{(B)}}{\mathop{\text{product}}}\,\]
A)
\[A:{{H}_{3}}C-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\] \[B:{{H}_{3}}C-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}\]
done
clear
B)
\[A:{{H}_{3}}C-\underset{S{{O}_{4}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\] \[B:{{H}_{3}}C-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,=C{{H}_{3}}\]
done
clear
C)
\[A:{{H}_{3}}C-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\] \[B:{{H}_{3}}C-\underset{S{{O}_{4}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\]
done
clear
D)
\[A:{{H}_{3}}C-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}\] \[B:{{H}_{3}}C-C\equiv CH\]
done
clear
View Answer play_arrow
question_answer 50) Which of the following reactions is zappropriate for converting acetamide to methanamine?
A)
Gabriels phthalimide synthesis
done
clear
B)
Carbylamine reaction
done
clear
C)
Hoffmann hypobromamide reaction
done
clear
D)
Stephens reaction
done
clear
View Answer play_arrow
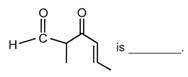
question_answer 51)
The IUPAC name of the compound
A)
3-keto-2-methylhex-5-enal
done
clear
B)
3-keto-2-methylhex-4-enal
done
clear
C)
5-formylhex-2-en-3-one
done
clear
D)
5-methyl-4-oxohex-2-en-5-al
done
clear
View Answer play_arrow
question_answer 52) Which of the following is a sink for CO?
A)
Plants
done
clear
B)
Haemoglobin
done
clear
C)
Micro-organisms present in the soil
done
clear
D)
Oceans
done
clear
View Answer play_arrow
question_answer 53) Of the following, which is the product formed when cyclohexanone undergoes aldol condensation followed by heating?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 54) Which of the following pairs of compounds is isoelectronic and isostructural?
A)
\[I{{F}_{3}},Xe{{F}_{2}}\]
done
clear
B)
\[BeC{{l}_{2}},Xe{{F}_{2}}\]
done
clear
C)
\[Te{{l}_{2}},Xe{{F}_{2}}\]
done
clear
D)
\[IBr_{2}^{-},Xe{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 55)
Consider the reactions:
A)
A-Ethanol, X-Acetaldehyde, Y-Butanone, Z-Hydrazone
done
clear
B)
A-Methoxymethane, X-Ethanoic acid, Y-Acetate ion, Z-hydrazine
done
clear
C)
A-Methoxymethane, X-Ethanol, Y-Ethanoic acid, Z-Semicarbazide
done
clear
D)
A-Ethanal, X-Ethanol, Y-But-2-enal, Z-Semicarbazone
done
clear
View Answer play_arrow
question_answer 56) Which one is the most acidic compound?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 57) Name the gas that can readily decolourises acidified\[KMn{{O}_{4}}\]solution:
A)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 58) Which one is the correct order of acidity?
A)
\[C{{H}_{3}}=C{{H}_{3}}>C{{H}_{2}}=C{{H}_{2}}>C{{H}_{3}}-C=CH>CH=CH\]
done
clear
B)
\[C{{H}_{2}}=C{{H}_{2}}>C{{H}_{3}}-CH=C{{H}_{2}}>C{{H}_{3}}-C\equiv CH>\]\[CH\equiv CH\]
done
clear
C)
\[CH\equiv CH>C{{H}_{3}}-C\equiv CH>C{{H}_{2}}=C{{H}_{2}}>C{{H}_{3}}-C{{H}_{3}}\]
done
clear
D)
\[CH\equiv CH>C{{H}_{2}}=C{{H}_{2}}>C{{H}_{3}}-C\equiv CH>C{{H}_{3}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 59) Concentration of the\[A{{g}^{+}}\]ions in a saturated solution of \[A{{g}_{2}}{{C}_{2}}{{O}_{4}}\]is \[2.2\times {{10}^{-4}}\,mol\,{{L}^{-1}}.\]Solubility product of \[A{{g}_{2}}{{C}_{2}}{{O}_{4}}\]is
A)
\[5.3\times {{10}^{12}}\]
done
clear
B)
\[~2.42\times {{10}^{8}}\]
done
clear
C)
\[2.66\times {{10}^{12}}\]
done
clear
D)
\[~4.5\times {{10}^{11}}\]
done
clear
View Answer play_arrow
question_answer 60) With respect to the conformers of ethane, which of the following statements is true?
A)
Both bond angles and bond length remains same
done
clear
B)
Bond angle remains same but bond length changes
done
clear
C)
Bond angle changes but bond length remains
done
clear
D)
Both bond angle and bond length change
done
clear
View Answer play_arrow
question_answer 61) The correct statement regarding electrophile is
A)
Electrophile can be either neutral or positively charged species and can form a bond by accepting a pair of electrons from a nucleophile
done
clear
B)
Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from a nucleophile
done
clear
C)
Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from another electrophile
done
clear
D)
Electrophiles are generally neutral species and can form a bond by accepting a pair of electrons from a nucleophile
done
clear
View Answer play_arrow
question_answer 62) Which one is the wrong statement?
A)
The energy of 2s orbital is less than the energy of 2p orbital in case of Hydrogen like atoms
done
clear
B)
de-Broglie's wavelength is given by \[\lambda =\frac{h}{mv},\] where m = mass of the particle, v = group velocity of the particle
done
clear
C)
The uncertainty principle is \[\Delta E\times \Delta t\ge \frac{h}{4\pi }\]
done
clear
D)
Half-filled and fully filled orbitals have greater stability due to greater exchange energy, greater symmetry and more balanced arrangement
done
clear
View Answer play_arrow
question_answer 63) Correct increasing order for the wavelengths of absorption in the visible region for the complexes of \[C{{o}^{3+}}\]is
A)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(en)}_{3}}]}^{3+}},{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
B)
\[{{[Co{{(en)}_{3}}]}^{3+}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}},{{[Co{{(en)}_{3}}]}^{3+}},{{[Co{{(N{{H}_{3}}O)}_{6}}]}^{3+}}\]
done
clear
D)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{3+}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(e{{n}_{3}})}_{3}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 64)
Match the inter halogen compounds of column I with the geometry in column II and assign the correct code Column I Column II A. \[XX'\] (i) T-shape B. \[XX_{3}^{'}\] (ii) Pentagonal bipyramidal C. \[XX_{5}^{'}\] (iii) Linear D. \[XX_{7}^{'}\] (iv) Square-pyramidal (v) Tetrahedral
A)
A-iv B-iii C-ii D-i
done
clear
B)
A-iii B-iv C-i D-ii
done
clear
C)
A-iii B-i C-iv D-ii
done
clear
D)
A-v B-iv C-iii D-ii
done
clear
View Answer play_arrow
question_answer 65) The species, having bond angles of \[{{120}^{o}}\] is
A)
\[BC{{l}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[CI{{F}_{3}}\]
done
clear
D)
\[NC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 66)
The correct increasing order of basic strength for the following compounds is
A)
II < I < III
done
clear
B)
II < III < I
done
clear
C)
III < I < II
done
clear
D)
III < II < I
done
clear
View Answer play_arrow
question_answer 67) Which one of the following statements is not correct?
A)
Coenzymes increase the catalytic activity of enzyme
done
clear
B)
Catalyst does not initiate any reaction
done
clear
C)
The value of equilibrium constant is changed in the presence of a catalyst in the reaction at equilibrium
done
clear
D)
Enzymes catalyse mainly bio-chemical reactions
done
clear
View Answer play_arrow
question_answer 68) A gas is allowed to expand in a well-insulated container against a constant external pressure of 2.5 atm from an initial volume of 2.50 L to a final volume of 4.50 L. The change in internal energy \[\Delta U\] of the gas in joules will be
A)
+505 J
done
clear
B)
1136.25 J
done
clear
C)
-500 J
done
clear
D)
-505 J
done
clear
View Answer play_arrow
question_answer 69) A 20 litre container at 400 K contains \[C{{O}_{2}}(g)\]pressure 0.4 atm and an excess of SrO (neglect the volume of solid SrO). The volume of the containers is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when pressure of \[C{{O}_{2}}\]attains its maximum value, will be (Given that:\[SrC{{O}_{3}}(s)=SrO(s)+C{{O}_{2}}(g).\]\[{{K}_{p}}=1.6\,\text{atm})\]
A)
2 litre
done
clear
B)
5 litre
done
clear
C)
10 litre
done
clear
D)
4 litre
done
clear
View Answer play_arrow
question_answer 70) Which of the following statements is not correct?
A)
Denaturation makes the proteins more active
done
clear
B)
Insulin maintains sugar level in the blood of a human body
done
clear
C)
Ovalbumin is a simple food reserve in egg-white
done
clear
D)
Blood proteins thrombin and fibrinogen are involved in blood clotting
done
clear
View Answer play_arrow
question_answer 71)
Mechanism of a hypothetical reaction\[{{X}_{2}}+{{Y}_{2}}\to 2XY\] is given below: (i) \[{{X}_{2}}\to X+X(fast)\] (ii) \[X+{{Y}_{2}}XY+Y(slow)\] (iii)\[X+Y\to XY(fast)\]
The overall order of the reaction will be
A)
1.5
done
clear
B)
1
done
clear
C)
2
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 72) In which pair of ions both the species contain S-S bond?
A)
\[{{S}_{4}}O_{6}^{2-},{{S}_{2}}O_{7}^{2-}\]
done
clear
B)
\[{{S}_{2}}O_{7}^{2-},{{S}_{2}}O_{3}^{2-}\]
done
clear
C)
\[{{S}_{4}}O_{6}^{2-},{{S}_{2}}O_{3}^{2-}\]
done
clear
D)
\[{{S}_{2}}O_{7}^{2-},{{S}_{2}}O_{8}^{2-}\]
done
clear
View Answer play_arrow
question_answer 73) Which one of the following pairs of species have the same bond order?
A)
\[{{N}_{2}}O_{2}^{-}\]
done
clear
B)
\[CO,NO\]
done
clear
C)
\[{{O}_{2}},N{{O}^{+}}\]
done
clear
D)
\[C{{N}^{-}},CO\]
done
clear
View Answer play_arrow
question_answer 74) Mixture of chloroxylenol and terpineol acts as
A)
Antibiotic
done
clear
B)
Analgesic
done
clear
C)
Antiseptic
done
clear
D)
Antipyretic
done
clear
View Answer play_arrow
question_answer 75) It is because of inability of\[n{{s}^{2}}\]electrons of the valence shell to participate in bonding that
A)
\[S{{n}^{4+}}\] is reducing while \[P{{b}^{4+}}\] is oxidizing
done
clear
B)
\[S{{n}^{2+}}\]is reducing while \[P{{b}^{4+}}\]is oxidizing
done
clear
C)
\[S{{n}^{2+}}\] is oxidising while \[P{{b}^{4+}}\]is reducing
done
clear
D)
\[S{{n}^{2+}}\]and\[P{{b}^{2+}}\]are both oxidising and reducing
done
clear
View Answer play_arrow
question_answer 76) For a given reaction,\[\Delta =35.5\,kJ\,mo{{l}^{-1}}\] and \[\Delta S=83.6\,J{{K}^{-1}}\,mo{{l}^{-1}}.\]The reaction is spontaneous at : (Assume that\[\Delta H\]and\[\Delta S\] do not vary with temperature)
A)
T > 298 K
done
clear
B)
T < 425 K
done
clear
C)
T > 425 K
done
clear
D)
All temperatures
done
clear
View Answer play_arrow
question_answer 77) If molality of the dilute solution is doubled, the value of molal depression constant \[({{K}_{f}})\]will be
A)
Unchanged
done
clear
B)
Doubled
done
clear
C)
Halved
done
clear
D)
Tripled
done
clear
View Answer play_arrow
question_answer 78) Which of the following is dependent on temperature?
A)
Weight percentage
done
clear
B)
Molality
done
clear
C)
Molarity
done
clear
D)
Mole fraction
done
clear
View Answer play_arrow
question_answer 79) Pick out the correct statement with respect \[{{[Mn{{(CN)}_{6}}]}^{3-}}\]
A)
It is \[ds{{p}^{2}}\]hybridised and square planar
done
clear
B)
It is \[s{{p}^{3}}{{d}^{2}}\]hybridised and octahedral
done
clear
C)
It is \[s{{p}^{3}}{{d}^{2}}\] hybridised and tetrahedral
done
clear
D)
It is\[{{d}^{2}}s{{p}^{3}}\] hybridised and octahedral
done
clear
View Answer play_arrow
question_answer 80) Which is the incorrect statement?
A)
Frenkel defect is favoured in those ionic compounds in which sizes of cation and anions are almost equal
done
clear
B)
\[Fe{{O}_{0.98}}\] has non stoichiometric metal deficiency defect
done
clear
C)
Density decreases in case of crystals with Schottky's defect
done
clear
D)
\[NaCl(s)\]is insulator, silicon is semiconductor, silver is conductor, quartz is piezo electric crystal
done
clear
View Answer play_arrow
question_answer 81) \[HgC{{l}_{2}}\]and \[{{l}_{2}}\] both when dissolved in water containing \[{{l}^{-}}\]ions the pair of species formed is
A)
\[H{{g}_{2}}{{l}_{2}},{{l}^{-}}\]
done
clear
B)
\[Hg{{l}_{2}},l_{3}^{-}\]
done
clear
C)
\[Hg{{l}_{2}},{{l}^{-}}\]
done
clear
D)
\[Hgl_{4}^{2-},l_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 82) Extraction of gold and silver involves leaching with \[C{{N}^{-}}\]ion. Silver is later recovered by
A)
Displacement with Zn
done
clear
B)
Liquation
done
clear
C)
Distillation
done
clear
D)
Zone refining
done
clear
View Answer play_arrow
question_answer 83) The correct order of the stoichiometries of AgCl formed when\[AgN{{O}_{3}}\]in excess is treated with the complexes :\[CoC{{l}_{3}}.6N{{H}_{3}},CoC{{l}_{3}}.5N{{H}_{3}},CoC{{l}_{3}}.4N{{H}_{3}}\] respectively is
A)
\[2\,AgCl,3AgCl,\,1\,AgCl\]
done
clear
B)
\[1\,AgCl,3AgCl,\,2\,AgCl\]
done
clear
C)
\[3\,AgCl,1AgCl,\,2\,AgCl\]
done
clear
D)
\[3\,AgCl,2AgCl,\,1\,AgCl\]
done
clear
View Answer play_arrow
question_answer 84)
Identify A and predict the type of reaction
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 85) An example of a sigma bonded organometallic compound is
A)
Cobaltocene
done
clear
B)
Ruthenocene
done
clear
C)
Grignard's reagent
done
clear
D)
Ferrocene
done
clear
View Answer play_arrow
question_answer 86) The reason for greater range of oxidation states in actinoids is attributed to
A)
4f and 5d levels being close in energies
done
clear
B)
The radioactive nature of actinoids
done
clear
C)
Actinoid contraction
done
clear
D)
5f, 6d and 7s levels having comparable energies
done
clear
View Answer play_arrow
question_answer 87) The element Z = 114 has been discovered recently. It will belong to which of the following family group and electronic configuration?
A)
Nitrogen family, \[[Rn]5{{f}^{14}}6{{d}^{10}}7{{s}^{2}}7{{p}^{6}}\]
done
clear
B)
Halogen family, \[[Rn]5{{f}^{14}}6{{d}^{10}}7{{s}^{2}}7{{p}^{5}}\]
done
clear
C)
Carbon family, \[[Rn]5{{f}^{14}}6{{d}^{10}}7{{s}^{2}}7{{p}^{2}}\]
done
clear
D)
Oxygen family,\[[Rn]5{{f}^{14}}6{{d}^{10}}7{{s}^{2}}7{{p}^{4}}\]
done
clear
View Answer play_arrow
question_answer 88) A first order reaction has a specific reaction rate of\[{{10}^{-2}}{{s}^{-1}}.\] How much time will it take for 20 g of the reactant to reduce to 5 g?
A)
693.0 second
done
clear
B)
238.6 second
done
clear
C)
138.6 second
done
clear
D)
346.5 second
done
clear
View Answer play_arrow
question_answer 89) Ionic mobility of which of the following alkali metal ions is lowest when aqueous solution of their salts are put under an electric field?
A)
Li
done
clear
B)
Na
done
clear
C)
K
done
clear
D)
Rb
done
clear
View Answer play_arrow
question_answer 90) In the electrochemical cell \[Zn|ZnS{{O}_{4}}(0.01)||CuS{{O}_{4}}(1.0\,M)Cu,\]the emf of this Daniel cell is\[{{E}_{1}}.\]When the concentration of \[ZnS{{O}_{4}}\]is changed to 1.0 M and that of \[CuS{{O}_{4}}\]changed to 0.01 M, emf changes to\[{{E}_{2}}.\]From the following, which one is the relationship between\[{{E}_{1}}\]and\[{{E}_{2}}\]? (Given, \[\frac{RT}{F}=0.059\])
A)
\[{{E}_{2}}=0\ne {{E}_{1}}\]
done
clear
B)
\[{{E}_{1}}={{E}_{2}}\]
done
clear
C)
\[{{E}_{1}}<{{E}_{2}}\]
done
clear
D)
\[{{E}_{1}}>{{E}_{2}}\]
done
clear
View Answer play_arrow
question_answer 91) The final proof for DNA as the genetic material came from the experiments of
A)
Hargobind Khorana
done
clear
B)
Griffith
done
clear
C)
Hershey and Chase
done
clear
D)
Avery, Mcleod and McCarty
done
clear
View Answer play_arrow
question_answer 92) Spliceosomes are not found in cells of
A)
Bacteria
done
clear
B)
Plants
done
clear
C)
Fungi
done
clear
D)
Animals
done
clear
View Answer play_arrow
question_answer 93) The pivot joint between atlas and axis is a type of
A)
Saddle joint
done
clear
B)
Fibrous joint
done
clear
C)
Cartilaginous joint
done
clear
D)
Synovial joint
done
clear
View Answer play_arrow
question_answer 94) The association of histone H1 with a nucleosome indicates:
A)
The DNA double helix is exposed
done
clear
B)
Transcription is occurring
done
clear
C)
DNA replication is occurring
done
clear
D)
The DNA is condensed into a Chromatin Fibre
done
clear
View Answer play_arrow
question_answer 95) Therefore the DNA is in condensed form. Which of the following is made up of dead cells?
A)
Phloem
done
clear
B)
Xylem parenchyma
done
clear
C)
Collenchyma
done
clear
D)
Phellem
done
clear
View Answer play_arrow
question_answer 96) Select the correct route for the passage of sperms in male frogs :
A)
Testes\[\to \]Vasa efferentia\[\to \]Kidney\[\to \] Bidder's canal \[\to \] Urinogenital duct\[\to \]Cloaca
done
clear
B)
Testes\[\to \]Bidder's canal\[\to \]Kidney\[\to \]Vasa efferentia\[\to \]Urinogenital duct \[\to \]Cloaca
done
clear
C)
Testes \[\to \]Vasa efferentia\[\to \]Kidney\[\to \] Seminal Vesicle \[\to \]Urinogenital duct \[\to \]Cloaca
done
clear
D)
Testes \[\to \]Vasa efferentia\[\to \]Bidder's canal \[\to \] Ureter\[\to \]Cloaca
done
clear
View Answer play_arrow
question_answer 97)
Adult human RBCs are enucleate. Which of the following statement(s) is/are most appropriate explanation for this feature?
[a] They do not need to reproduce
[b] They are somatic cells
[c] They do not metabolize
[d] All their internal space is available for oxygen transport
A)
[b] and [c]
done
clear
B)
Only [d]
done
clear
C)
Only [a]
done
clear
D)
[a], [c] and [d]
done
clear
View Answer play_arrow
question_answer 98) Homozygous purelines in cattle can be obtained by
A)
Mating of individuals of different species
done
clear
B)
Mating of related individuals of same breed
done
clear
C)
Mating of unrelated individuals of same breed
done
clear
D)
Mating of individuals of different breed
done
clear
View Answer play_arrow
question_answer 99) A temporary endocrine gland in the human body is
A)
Corpus allatum
done
clear
B)
Pineal gland
done
clear
C)
Corpus cardiacum
done
clear
D)
Corpus luteum
done
clear
View Answer play_arrow
question_answer 100) Viroids differ from viruses in having :
A)
RNA molecules without protein coat
done
clear
B)
DNA molecules with protein coat
done
clear
C)
DNA molecules without protein coat
done
clear
D)
RNA molecules with protein coat
done
clear
View Answer play_arrow
question_answer 101) A decrease in blood pressure/volume will not cause the release of
A)
ADH
done
clear
B)
Renin
done
clear
C)
Atrial Natriuretic Factor
done
clear
D)
Aldosterone
done
clear
View Answer play_arrow
question_answer 102) An example of colonial alga is
A)
Spirogyra
done
clear
B)
Chlorella
done
clear
C)
Volvox
done
clear
D)
Ulothrix
done
clear
View Answer play_arrow
question_answer 103) The morphological nature of the edible part of coconut is
A)
Pericarp
done
clear
B)
Perisperm
done
clear
C)
Cotyledon
done
clear
D)
Endosperm
done
clear
View Answer play_arrow
question_answer 104) Which of the following is correctly matched for the product produced by them?
A)
Saccharomyces cerevisiae : Ethanol
done
clear
B)
Acetobacter aceti : Antibiotics
done
clear
C)
Methanobacterium : Lactic acid
done
clear
D)
Penicillium notatum : Acetic acid
done
clear
View Answer play_arrow
question_answer 105)
Match the following sexually transmitted diseases (Column - I) with their causative agent (Column - II) and select the correct option. Column - I Column- II [A] Gonorrhea (i) HIV [B] Syphilis (ii) Neisseria [C] Genital Warts (iii) Treponema [D] AIDS (iv) Human Papilloma virus
A)
A-iv B-iii C-ii D-i
done
clear
B)
A-ii B-iii C-iv D-i
done
clear
C)
A-iii B-iv C-i D-ii
done
clear
D)
A-iv B-ii C-iii D-i
done
clear
View Answer play_arrow
question_answer 106) In case of poriferans the spongocoel is lined with flagellated cells called :
A)
Mesenchymal cells
done
clear
B)
Ostia
done
clear
C)
Oscula
done
clear
D)
Choanocytes
done
clear
View Answer play_arrow
question_answer 107) Among the following characters, which one was not considered by Mendel in his experiments on pea?
A)
Pod - Inflated or Constricted
done
clear
B)
Stem - Tall or Dwarf
done
clear
C)
Trichomes - Glandular or non-glandular
done
clear
D)
Seed - Green or Yellow
done
clear
View Answer play_arrow
question_answer 108) Identify the wrong statement in context of heartwood.
A)
It comprises dead elements with highly lignified walls
done
clear
B)
Organic compounds are deposited in it
done
clear
C)
It is highly durable
done
clear
D)
It conducts water and minerals efficiently
done
clear
View Answer play_arrow
question_answer 109) During DNA replication, Okazaki fragments are used to enlongate
A)
The lagging strand away from the replication fork
done
clear
B)
The leading strand towards replication fork
done
clear
C)
The lagging strand towards replication fork
done
clear
D)
The leading strand away from replication fork
done
clear
View Answer play_arrow
question_answer 110) Mycorrhizae are the example of
A)
Mutualism
done
clear
B)
Fungistasis
done
clear
C)
Amensalism
done
clear
D)
Antibiosis
done
clear
View Answer play_arrow
question_answer 111) Which of the following RNAs should be most abundant in animal cell?
A)
mi-RNA
done
clear
B)
r-RNA
done
clear
C)
t-RNA
done
clear
D)
m-RNA
done
clear
View Answer play_arrow
question_answer 112) The process of separation and purification of expressed protein before marketing is called
A)
Postproduction processing
done
clear
B)
Upstream processing
done
clear
C)
Downstream processing
done
clear
D)
Bioprocessing
done
clear
View Answer play_arrow
question_answer 113) Which among the following are the smallest living cells, known without a definite cell wall, pathogenic to plants as well as animals and can survive without oxygen?
A)
Nostoc
done
clear
B)
Bacillus
done
clear
C)
Pseudomonas
done
clear
D)
Mycoplasma
done
clear
View Answer play_arrow
question_answer 114) Which of the following components provides sticky character to the bacterial cell?
A)
Glycocalyx
done
clear
B)
Cell wall
done
clear
C)
Nuclear membrane
done
clear
D)
Plasma membrane
done
clear
View Answer play_arrow
question_answer 115) With reference to factors affecting the rate of photosynthesis, which of the following statements is not correct?
A)
Tomato is a greenhouse crop which can be grown in \[C{{O}_{2}}-\]enriched atmosphere for higher yield
done
clear
B)
Light saturation for\[C{{O}_{2}}\] fixation occurs at 10% of full sunlight
done
clear
C)
Increasing atmospheric \[C{{O}_{2}}\] concentration upto 0.05% can enhance \[C{{O}_{2}}\] fixation rate
done
clear
D)
\[{{C}_{3}}\] plants responds to higher temperatures with enhanced photosynthesis while \[{{C}_{4}}\] plants have much lower temperature optimum
done
clear
View Answer play_arrow
question_answer 116) Which of the following options best represents the enzyme composition of pancreatic juice?
A)
Lipase, amylase, trypsinogen, procarboxy-peptidase
done
clear
B)
Amylase, peptidase, trypsinogen, rennin
done
clear
C)
Amylase, pepsin, trypsinogen, maltase
done
clear
D)
Peptidase, amylase, pepsin, rennin
done
clear
View Answer play_arrow
question_answer 117) Which one of the following statements is correct, with reference to enzymes?
A)
Holoenzyme = Coenzyme + Cofactor
done
clear
B)
Apoenzyme = Holoenzyme + Coenzyme
done
clear
C)
Holoenzyme = Apoenzyme + Coenzyme
done
clear
D)
Coenzyme = Apoenzyme + Holoenzyme
done
clear
View Answer play_arrow
question_answer 118) If there are 999 bases in an RNA that codes for a protein with 333 amino acids, and the base at position 901 is deleted such that the length of the RNA becomes 998 bases, how many codons will be altered?
A)
333
done
clear
B)
1
done
clear
C)
11
done
clear
D)
33
done
clear
View Answer play_arrow
question_answer 119) Asymptote in a logistic growth curve is obtained when
A)
K < N
done
clear
B)
The value of 'r' approaches zero
done
clear
C)
K = N
done
clear
D)
K > N
done
clear
View Answer play_arrow
question_answer 120) Select the mismatch:
A)
Equisetum - Homosporous
done
clear
B)
Pinus - Dioecious
done
clear
C)
Cycas - Dioecious
done
clear
D)
Salvinia - Heterosporous
done
clear
View Answer play_arrow
question_answer 121) Anaphase promoting complex (APC) is a protein degradation machinery necessary for proper mitosis of animal cells. If APC is defective in a human cell, which of the following is expected to occur?
A)
Recombination of chromosome arms will occur
done
clear
B)
Chromosomes will not condense
done
clear
C)
Chromosomes will be fragmented
done
clear
D)
Chromosomes will not segregate
done
clear
View Answer play_arrow
question_answer 122) Which ecosystem has the maximum biomass?
A)
Lake ecosystem
done
clear
B)
Forest ecosystem
done
clear
C)
Grassland ecosystem
done
clear
D)
Pond ecosystem
done
clear
View Answer play_arrow
question_answer 123) Zygotic meiosis is characterstic of
A)
Chlamydomonas
done
clear
B)
Marchantia
done
clear
C)
Fucus
done
clear
D)
Funaria
done
clear
View Answer play_arrow
question_answer 124) Hypersecretion of Growth Hormone in adults does not cause further increase in height, because
A)
Muscle fibres do not grow in size after birth
done
clear
B)
Growth Hormone becomes inactive in adults
done
clear
C)
Epiphyseal plates close after adolescence
done
clear
D)
Bones loose their sensitivity to Growth Hormone in adults
done
clear
View Answer play_arrow
question_answer 125)
Frog's heart when taken out of the body continues to beat for some time Select the best option from the following statements [a] Frog is a poikilotherm [b] Frog does not have any coronary circulation [c] Heart is "myogenic" in nature [d] Heart is autoexcitable
Options:
A)
[c] & [d]
done
clear
B)
Only [c]
done
clear
C)
Only [d]
done
clear
D)
[a] & [b]
done
clear
View Answer play_arrow
question_answer 126) Transplantation of tissues/organs fails often due to non-acceptance by the patient's body. Which type of immune-response is responsible for such rejections?
A)
Physiological immune response
done
clear
B)
Autoimmune response
done
clear
C)
Cell-mediated immune response
done
clear
D)
Hormonal immune response
done
clear
View Answer play_arrow
question_answer 127) Thalassemia and sickle cell anemia are caused due to a problem in globin molecule synthesis. Select the correct statement.
A)
Sickle cell anemia is due to a quantitative problem of globin molecules
done
clear
B)
Both are due to a qualitative defect in globin chain synthesis
done
clear
C)
Both are due to a quantitative defect in globin chain synthesis
done
clear
D)
Thalassemia is due to less synthesis of globin molecules
done
clear
View Answer play_arrow
question_answer 128) An important characteristic that Hemichordates share with Chordates is
A)
Pharynx without gill slits
done
clear
B)
Absence of notochord
done
clear
C)
Ventral tubular nerve cord
done
clear
D)
Pharynx with gill slits
done
clear
View Answer play_arrow
question_answer 129) Double fertilization is exhibited by
A)
Angiosperms
done
clear
B)
Gymnosperms
done
clear
C)
Algae
done
clear
D)
Fungi
done
clear
View Answer play_arrow
question_answer 130) Which of the following cell organelles is responsible for extracting energy from carbohydrates to form ATP?
A)
Mitochondrion
done
clear
B)
Lysosome
done
clear
C)
Ribosome
done
clear
D)
Chloroplast
done
clear
View Answer play_arrow
question_answer 131) Lungs are made up of air-filled sacs the alveoli. They do not collapse even after forceful expiration, because of:
A)
Expiratory Reserve Volume
done
clear
B)
Residual Volume
done
clear
C)
Inspiratory Reserve Volume
done
clear
D)
Tidal Volume
done
clear
View Answer play_arrow
question_answer 132) Which of the following are not polymeric?
A)
Lipids
done
clear
B)
Nucleic acids
done
clear
C)
Proteins
done
clear
D)
Polysaccharides
done
clear
View Answer play_arrow
question_answer 133) Flowers which have single ovule in the ovary and are packed into inflorescence are usually pollinated by
A)
Bat
done
clear
B)
Water
done
clear
C)
Bee
done
clear
D)
Wind
done
clear
View Answer play_arrow
question_answer 134) Life cycle of Ectocarpus and Fucus respectively are
A)
Haplodiplontic, Haplontic
done
clear
B)
Haplontic, Diplontic
done
clear
C)
Diplontic, Haplodiplontic
done
clear
D)
Haplodiplontic, Diplontic
done
clear
View Answer play_arrow
question_answer 135) Presence of plants arranged into well-defined vertical layers depending on their height can be seen best in :
A)
Temperate Forest
done
clear
B)
Tropical Savannah
done
clear
C)
Tropical Rain Forest
done
clear
D)
Grassland
done
clear
View Answer play_arrow
question_answer 136) Phosphonol pyruvate (PEP) is the primary \[C{{O}_{2}}\] acceptor in :
A)
\[{{C}_{3}}\]and \[{{C}_{4}}\]plants
done
clear
B)
\[{{C}_{3}}\]plants
done
clear
C)
\[{{C}_{4}}\]plants
done
clear
D)
\[{{C}_{2}}\]plants
done
clear
View Answer play_arrow
question_answer 137)
Good vision depends on adequate intake of carotene rich food Select the best option from the following statements [a] Vitamin A derivatives are formed from carotene [b] The photopigments are embedded in the membrane discs of the inner segment [c] Retinal is a derivative of vitamin A [d] Retinal is a light absorbing part of all the visual photo pigments
Options:
A)
[b], [c] & [d]
done
clear
B)
[a] & [b]
done
clear
C)
[a], [c] & [d]
done
clear
D)
[a] & [c]
done
clear
View Answer play_arrow
question_answer 138) Which one from those given below is the period for Mendel's hybridization experiments?
A)
1870 - 1877
done
clear
B)
1856 - 1863
done
clear
C)
1840 - 1850
done
clear
D)
1857 - 1869
done
clear
View Answer play_arrow
question_answer 139) Select the mismatch :
A)
Rhizobium - Alfalfa
done
clear
B)
Frankia - Alnus
done
clear
C)
Rhodospirillum - Mycorrhiza
done
clear
D)
Anabaena - Nitrogen fixer
done
clear
View Answer play_arrow
question_answer 140) Attractants and rewards are required for
A)
Cleistogamy
done
clear
B)
Anemophily
done
clear
C)
Entomophily
done
clear
D)
Hydrophily
done
clear
View Answer play_arrow
question_answer 141) In case of a couple where the male is having a very low sperm count, which technique will be suitable for fertilisation?
A)
Intra cytoplasmic sperm injection
done
clear
B)
Intrauterine transfer
done
clear
C)
Gamete intra cytoplasmic fallopian transfer
done
clear
D)
Artificial Insemination
done
clear
View Answer play_arrow
question_answer 142) Which among these is the correct combination of aquatic mammals?
A)
Trygon, Whales, Seals
done
clear
B)
Seals, Dolphins, Sharks
done
clear
C)
Dolphins, Seals, Trygon
done
clear
D)
Whales, Dolphins Seals
done
clear
View Answer play_arrow
question_answer 143) Functional megaspore in an angiosperm develops into
A)
Embryo
done
clear
B)
Ovule
done
clear
C)
Endosperm
done
clear
D)
Embryo sac
done
clear
View Answer play_arrow
question_answer 144) Root hairs develop from the region of
A)
Meristematic activity
done
clear
B)
Maturation
done
clear
C)
Elongation
done
clear
D)
Root cap
done
clear
View Answer play_arrow
question_answer 145) A dioecious flowering plant prevents both:
A)
Cleistogamy and xenogamy
done
clear
B)
Autogamy and xenogamy
done
clear
C)
Autogamy and geitonogamy
done
clear
D)
Geitonogamy and xenogamy
done
clear
View Answer play_arrow
question_answer 146) The hepatic portal vein drains blood to liver from
A)
Intestine
done
clear
B)
Heart
done
clear
C)
Stomach
done
clear
D)
Kidneys
done
clear
View Answer play_arrow
question_answer 147) What is the criterion for DNA fragments movement on agarose gel during gel electrophoresis?
A)
Negatively charged fragments do not move
done
clear
B)
The larger the fragment size, the farther it moves
done
clear
C)
The smaller the fragment size, the farther it moves
done
clear
D)
Positively charged fragments move to farther end
done
clear
View Answer play_arrow
question_answer 148) Which of the following represents order of 'Horse'?
A)
Ferus
done
clear
B)
Equidae
done
clear
C)
Perissodactyla
done
clear
D)
Caballus
done
clear
View Answer play_arrow
question_answer 149) Which statement is wrong for Krebs' cycle?
A)
The cycle starts with condensation of acetyl group (acetyl CoA) with pyruvic acid to yield citric acid
done
clear
B)
There are three points in the cycle where \[NA{{D}^{+}}\]is reduced to \[NADH+{{H}^{+}}\]
done
clear
C)
There is one point in the cycle where \[FA{{D}^{+}}\]is reduced to \[FAD{{H}_{2}}\]
done
clear
D)
During conversion of succinyl CoA to succinic acid, a molecule of GTP is synthesized
done
clear
View Answer play_arrow
question_answer 150) Artificial selection to obtain cows yielding higher milk output represents
A)
Stabilizing followed by disruptive as it stabilizes the population to produce higher yielding cows
done
clear
B)
Stabilizing selection as it stabilizes this character in the population
done
clear
C)
Directional as it pushes the mean of the character in one direction
done
clear
D)
Disruptive as it splits the population into two one yielding higher output and the other lower output
done
clear
View Answer play_arrow
question_answer 151) The region of Biosphere Reserve which is legally protected and where no human activity is allowed is known as
A)
Restoration zone
done
clear
B)
Core zone
done
clear
C)
Buffer zone
done
clear
D)
Transition zone
done
clear
View Answer play_arrow
question_answer 152) Receptor sites for neurotransmitters are present on
A)
Post-synaptic membrane
done
clear
B)
Membranes of synaptic vesicles
done
clear
C)
Pre-synaptic membrane
done
clear
D)
Tips of axons
done
clear
View Answer play_arrow
question_answer 153) The vascular cambium normally gives rise to
A)
Periderm
done
clear
B)
Phelloderm
done
clear
C)
Primary phloem
done
clear
D)
Secondary xylem
done
clear
View Answer play_arrow
question_answer 154) A baby boy aged two years is admitted to play school and passes through a dental check-up. The dentist observed that the boy had twenty teeth. Which teeth were absent?
A)
Molars
done
clear
B)
Incisors
done
clear
C)
Canines
done
clear
D)
Pre-molars
done
clear
View Answer play_arrow
question_answer 155) The water potential of pure water is
A)
More than one
done
clear
B)
Zero
done
clear
C)
Less than zero
done
clear
D)
More than zero but less than one
done
clear
View Answer play_arrow
question_answer 156) DNA fragments are
A)
Either positively or negatively charged depending on their size
done
clear
B)
Positively charged
done
clear
C)
Negatively charged
done
clear
D)
Neutral
done
clear
View Answer play_arrow
question_answer 157) Capacitation occurs in
A)
Female Reproductive tract
done
clear
B)
Rete testis
done
clear
C)
Epididymis
done
clear
D)
Vas deferens
done
clear
View Answer play_arrow
question_answer 158) The function of copper ions in copper releasing IUD's is :
A)
They inhibit ovulation
done
clear
B)
They suppress sperm motility and fertilizing capacity of sperms
done
clear
C)
They inhibit gametogenesis
done
clear
D)
They make uterus unsuitable for implantation
done
clear
View Answer play_arrow
question_answer 159) A gene whose expression helps to identify transformed cell is known as
A)
Structural gene
done
clear
B)
Selectable marker
done
clear
C)
Vector
done
clear
D)
Plasmid
done
clear
View Answer play_arrow
question_answer 160) Which one of the following statements is not valid for aerosols?
A)
They have negative impact on agricultural land
done
clear
B)
They are harmful to human health
done
clear
C)
They alter rainfall and monsoon patterns
done
clear
D)
They cause increased agricultural productivity
done
clear
View Answer play_arrow
question_answer 161) Which of the following statements is correct?
A)
The descending limb of loop of Henle is permeable to electrolytes
done
clear
B)
The ascending limb of loop of Henle is impermeable to water
done
clear
C)
The descending limb of loop of Henle is impermeable to water
done
clear
D)
The ascending limb of loop of Henle is permeable to water
done
clear
View Answer play_arrow
question_answer 162) Which of the following in sewage treatment removes suspended solids?
A)
Sludge treatment
done
clear
B)
Tertiary treatment
done
clear
C)
Secondary treatment
done
clear
D)
Primary treatment
done
clear
View Answer play_arrow
question_answer 163) GnRH, a hypothalamic hormone, needed in reproduction, acts on
A)
Posterior pituitary gland and stimulates secretion of LH and relaxin
done
clear
B)
Anterior pituitary gland and stimulates secretion of LH and oxytocin
done
clear
C)
Anterior pituitary gland and stimulates secretion of LH and FSH
done
clear
D)
Posterior pituitary gland and stimulates secretion of oxytocin and FSH
done
clear
View Answer play_arrow
question_answer 164) Which of the following facilitates opening of stomatal aperture?
A)
Longitudinal orientation of cellulose micro fibrils in the cell wall of guard cells
done
clear
B)
Contraction of outer wall of guard cells
done
clear
C)
Decrease in turgidity of guard cells
done
clear
D)
Radial orientation of cellulose micro fibrils in the cell wall of guard cells
done
clear
View Answer play_arrow
question_answer 165) The genotypes of a Husband and Wife are \[{{|}^{A}}{{|}^{B}}\]and\[{{|}^{A}}|.\] Among the blood types of their children, how many different genotypes and phenotypes are possible?
A)
4 genotypes ; 4 phenotypes
done
clear
B)
3 genotypes ; 3 phenotypes
done
clear
C)
3 genotypes ; 4 phenotypes
done
clear
D)
4 genotypes ; 3 phenotypes
done
clear
View Answer play_arrow
question_answer 166) Plants which produce characterstic pneumatophores and show vivipary belong to
A)
Hydrophytes
done
clear
B)
Mesophytes
done
clear
C)
Halophytes
done
clear
D)
Psammophytes
done
clear
View Answer play_arrow
question_answer 167) Alexander Von Humboldt described for the first time
A)
Population Growth equation
done
clear
B)
Ecological Biodiversity
done
clear
C)
Laws of limiting factor
done
clear
D)
Species area relationships
done
clear
View Answer play_arrow
question_answer 168) DNA replication in bacteria occurs
A)
Just before transcription
done
clear
B)
During S-phase
done
clear
C)
Within nucleolus
done
clear
D)
Prior to fission
done
clear
View Answer play_arrow
question_answer 169) MALT constitutes about------------ percent of the lymphoid tissue in human body
A)
10%
done
clear
B)
50%
done
clear
C)
20%
done
clear
D)
70%
done
clear
View Answer play_arrow
question_answer 170) In Bougainvillea thorns are the modifications of
A)
Leaf
done
clear
B)
Stipules
done
clear
C)
Adventitious root
done
clear
D)
Stem
done
clear
View Answer play_arrow
question_answer 171) Fruit and leaf drop at early stages can be prevented by the application of
A)
Gibberellic acid
done
clear
B)
Cytokinins
done
clear
C)
Ethylene
done
clear
D)
Auxins
done
clear
View Answer play_arrow
question_answer 172) Which of the following are found in extreme saline conditions?
A)
Mycobacteria
done
clear
B)
Archaebacteria
done
clear
C)
Eubacteria
done
clear
D)
Cyanobacteria
done
clear
View Answer play_arrow
question_answer 173) Coconut fruit is a
A)
Capsule
done
clear
B)
Drupe
done
clear
C)
Berry
done
clear
D)
Nut
done
clear
View Answer play_arrow
question_answer 174) The DNA fragments separated on an agarose gel can be visualised after staining with
A)
Ethidium bromide
done
clear
B)
Bromophenol blue
done
clear
C)
Acetocarmine
done
clear
D)
Aniline blue
done
clear
View Answer play_arrow
question_answer 175) Out of 'X' pairs of ribs in humans only 'Y' pairs are true ribs. Select the option that correctly represents values of X and Y and provides their explanation :
A)
X = 24, Y = 12 True ribs are dorsally attached to vertebral column but are free on ventral side
done
clear
B)
X = 12, Y = 7 True ribs are attached dorsally to vertebral column and ventrally to the sternum
done
clear
C)
X = 12, Y = 5 True ribs are attached dorsally to vertebral column and sternum on the two ends
done
clear
D)
X = 24, Y = 7 True ribs are dorsally attached to vertebral column but are free on ventral side
done
clear
View Answer play_arrow
question_answer 176) Myelin sheath is produced by
A)
Osteoclasts and Astrocytes
done
clear
B)
Schwann Cells and Oligodendrocytes
done
clear
C)
Astrocytes and Schwann Cells
done
clear
D)
Oligodendrocytes and Osteoclasts
done
clear
View Answer play_arrow
question_answer 177) Which of the following options gives the correct sequence of events during mitosis?
A)
Condensation\[\to \]arrangement at equator \[\to \]centromere division\[\to \]segregation\[\to \] telophase
done
clear
B)
Condensation\[\to \]nuclear membrane disassembly\[\to \]crossing over\[\to \]segregation\[\to \] telophase
done
clear
C)
Condensation\[\to \]nuclear membrane disassembly\[\to \]arrangement at equator\[\to \] centromere division\[\to \]segregation\[\to \] telophase
done
clear
D)
Condensation \[\to \]crossing over \[\to \]nuclear membrane disassembly\[\to \]segregation \[\to \] telophase
done
clear
View Answer play_arrow
question_answer 178) A disease caused by an autosomal primary non-disjunction is
A)
Sickle cell anemia
done
clear
B)
Down's syndrome
done
clear
C)
Klinefelter's syndrome
done
clear
D)
Turner's syndrome
done
clear
View Answer play_arrow
question_answer 179) Which cells of 'Crypts of Lieberkuhn' secrete antibacterial lysozyme?
A)
Kupffer cells
done
clear
B)
Argentaffin cells
done
clear
C)
Paneth cells
done
clear
D)
Zymogen cells
done
clear
View Answer play_arrow
question_answer 180) Which one of the following is related to Ex-situ conservation of threatened animals and plants?
A)
Himalayan region
done
clear
B)
Wildlife Safari parks
done
clear
C)
Biodiversity hot spots
done
clear
D)
Amazon rainforest
done
clear
View Answer play_arrow