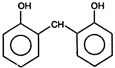
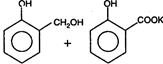
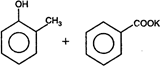
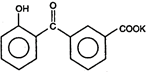
question_answer 1) Core of a transformer is made up of:
A)
soft iron
done
clear
B)
steel
done
clear
C)
iron
done
clear
D)
alnico
done
clear
View Answer play_arrow
question_answer 2) Transformer is based upon the principle of:
A)
self-induction
done
clear
B)
mutual induction
done
clear
C)
eddy current
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 3) Refractive index of material is equal to tangent of polarising angle. It is called:
A)
Brewsters law
done
clear
B)
Lamberts law
done
clear
C)
Malus law
done
clear
D)
Braggs law
done
clear
View Answer play_arrow
question_answer 4) A car accelerates from rest at constant rate for first \[10\,\,s\] and covers a distance\[x\]. It covers a distance \[y\] in next \[10\,\,s\] at the same acceleration. Which of the following is true?
A)
\[x=3y\]
done
clear
B)
\[y=3x\]
done
clear
C)
\[x=y\]
done
clear
D)
\[y=2x\]
done
clear
View Answer play_arrow
question_answer 5) The horizontal range of a projectile is\[400m\]. The maximum height attained by it will be:
A)
\[100m\]
done
clear
B)
\[200m\]
done
clear
C)
\[400m\]
done
clear
D)
\[800m\]
done
clear
View Answer play_arrow
question_answer 6) What determines the nature of the path followed by the particle?
A)
Speed
done
clear
B)
Velocity
done
clear
C)
Acceleration
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 7) Which of the following is not an example of perfectly inelastic collision?
A)
A bullet fired into a block if bullet gets embedded into block
done
clear
B)
Capture of electrons by an atom
done
clear
C)
A man jumping on to a moving boat
done
clear
D)
A ball bearing striking another ball bearing
done
clear
View Answer play_arrow
question_answer 8) The length of seconds pendulum is \[1\,\,m\] on earth. If mass and diameter of a planet is doubled than that of earth, then its length becomes:
A)
\[1\,\,m\]
done
clear
B)
\[2\,\,m\]
done
clear
C)
\[0.5\,\,m\]
done
clear
D)
\[4\,\,m\]
done
clear
View Answer play_arrow
question_answer 9) Why the dam of water reservoir is thick at the bottom?
A)
Quantity of water increases with depth
done
clear
B)
Density of water increases with depth
done
clear
C)
Pressure of water increases with depth
done
clear
D)
Temperature of water increases with depth
done
clear
View Answer play_arrow
question_answer 10) A hot and a cold body are kept in vacuum separated from each other. Which of the following causes decrease in temperature of the hot body?
A)
Radiation
done
clear
B)
Convection
done
clear
C)
Conduction
done
clear
D)
Temperature remains unchanged
done
clear
View Answer play_arrow
question_answer 11) When a tuning fork produces sound waves in air, which one of the following is same in the material of tuning fork as well as in air?
A)
Wavelength
done
clear
B)
Frequency
done
clear
C)
Velocity
done
clear
D)
Amplitude
done
clear
View Answer play_arrow
question_answer 12) A bomb explodes on the moon. How long will it take for the sound to reach the earth?
A)
\[10\,\,s\]
done
clear
B)
\[1000\,\,s\]
done
clear
C)
1 day
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 13) What is the angle between the electric dipole moment and the electric field strength due to it on the equatorial line?
A)
\[{{0}^{o}}\]
done
clear
B)
\[{{90}^{o}}\]
done
clear
C)
\[{{180}^{o}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 14) Which of the following is not the property of equipotential surfaces?
A)
They do not cross each other
done
clear
B)
They are concentric spheres for uniform electric field
done
clear
C)
Rate of change of potential with distance on them is zero
done
clear
D)
They can be imaginary spheres
done
clear
View Answer play_arrow
question_answer 15) The potentials of the two plates of capacitors are \[+10V\] and\[-10V\]. The charge on one of the plates is\[40\,\,C\]. The capacitance of the capacitor is:
A)
\[2\,\,F\]
done
clear
B)
\[4\,\,F\]
done
clear
C)
\[0.5\,\,F\]
done
clear
D)
\[0.25\,\,F\]
done
clear
View Answer play_arrow
question_answer 16) To draw maximum current from a combination of cells, how should the cells be grouped?
A)
Series
done
clear
B)
Parallel
done
clear
C)
Mixed
done
clear
D)
Depends upon the relative values of external and internal resistance
done
clear
View Answer play_arrow
question_answer 17) A wire is cut into four pieces, which are put together by sides to obtain one conductor. If the original resistance of wire was\[R\], the resistance of the bundle will be:
A)
\[\frac{R}{4}\]
done
clear
B)
\[\frac{R}{8}\]
done
clear
C)
\[\frac{R}{16}\]
done
clear
D)
\[\frac{R}{32}\]
done
clear
View Answer play_arrow
question_answer 18) Which of the following is not a property of light?
A)
It requires a material medium for propagation
done
clear
B)
It can travel through vacuum
done
clear
C)
It involves transportation of energy
done
clear
D)
It has finite speed
done
clear
View Answer play_arrow
question_answer 19) Which of the following pairs can produce erect, diminished and virtual image?
A)
Concave lens and convex mirror
done
clear
B)
Convex lens and convex mirror
done
clear
C)
Convex lens and concave mirror
done
clear
D)
Concave lens and concave mirror
done
clear
View Answer play_arrow
question_answer 20) For photoelectric emission, tungsten requires light of\[2300\overset{\text{o}}{\mathop{\text{A}}}\,\]. If light of \[1800\overset{\text{o}}{\mathop{\text{A}}}\,\] wavelength is incident then emission:
A)
takes place
done
clear
B)
doesnt take place
done
clear
C)
may or may not take place
done
clear
D)
depends on frequency
done
clear
View Answer play_arrow
question_answer 21) A type kept outside in sunlight bursts off after sometime because of:
A)
increase in pressure
done
clear
B)
increase in volume
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 22) For a wire of length Z, maximum change in length under stress condition is 2 mm. What is the charge in length under same conditions when length of wire is halved?
A)
\[1mm\]
done
clear
B)
\[2mm\]
done
clear
C)
\[4mm\]
done
clear
D)
\[8mm\]
done
clear
View Answer play_arrow
question_answer 23) Air is .blown through a hole on a closed pipe containing liquid. Then, the pressure will:
A)
increase on sides
done
clear
B)
increase downwards
done
clear
C)
increase in all directions
done
clear
D)
never increase
done
clear
View Answer play_arrow
question_answer 24) Light year is a unit of:
A)
time
done
clear
B)
speed
done
clear
C)
distance
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 25) A semiconductor doped with a donor impurity is:
A)
\[p-\]type
done
clear
B)
\[n-\]type
done
clear
C)
\[n-p-n\]type
done
clear
D)
\[p-n-p\]type
done
clear
View Answer play_arrow
question_answer 26) A wire carrying current I and other carrying\[2I\] in the same direction produce a magnetic field B at the mid-point. What will be the field when \[2I\] wire is switched off?
A)
\[B/2\]
done
clear
B)
\[2B\]
done
clear
C)
\[B\]
done
clear
D)
\[4B\]
done
clear
View Answer play_arrow
question_answer 27) What will be energy stored in a strained wire?
A)
\[\frac{1}{2}\times load\times extension\]
done
clear
B)
\[\frac{1}{2}\times stress\times strain\]
done
clear
C)
\[\frac{1}{2}\times load\times strain\]
done
clear
D)
\[\frac{1}{2}\times load\times stress\]
done
clear
View Answer play_arrow
question_answer 28) If error in measurement of radius of sphere is\[1%\], what will be the error in measurement of volume?
A)
\[1%\]
done
clear
B)
\[1/3%\]
done
clear
C)
\[3%\]
done
clear
D)
\[10%\]
done
clear
View Answer play_arrow
question_answer 29) Minimum number of unequal vectors which can give zero resultant are:
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
more than 4
done
clear
View Answer play_arrow
question_answer 30) A coin is dropped in a lift. If takes time \[{{t}_{1}}\] to reach the floor when lift is stationary. It takes time \[{{t}_{2}}\] when lift is moving up with constant acceleration. Then:
A)
\[{{t}_{1}}>{{t}_{2}}\]
done
clear
B)
\[{{t}_{2}}>{{t}_{1}}\]
done
clear
C)
\[{{t}_{1}}={{t}_{2}}\]
done
clear
D)
\[{{t}_{1}}>>{{t}_{2}}\]
done
clear
View Answer play_arrow
question_answer 31) A large ship can float but a steel needle sinks because of:
A)
viscosity
done
clear
B)
surface tension
done
clear
C)
density
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 32) In a solenoid, if number of turns is doubled, then self-inductance will become:
A)
half
done
clear
B)
double
done
clear
C)
1/4 times
done
clear
D)
quadruple
done
clear
View Answer play_arrow
question_answer 33) What causes change in the colours of the soap or oil films for the given beam of light?
A)
Angle of incidence
done
clear
B)
Angle of reflection
done
clear
C)
Thickness of film
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 34) What happens to the fringe pattern when the Youngs double slit experiment is performed in water instead of air?
A)
Shrinks
done
clear
B)
Disappear
done
clear
C)
Unchanged
done
clear
D)
Enlarged
done
clear
View Answer play_arrow
question_answer 35) Sir C.V. Raman was awarded Nobel Prize for his work concerned with which of the following phenomena of radiation?
A)
Scattering
done
clear
B)
Diffraction
done
clear
C)
Interference
done
clear
D)
Polarisation
done
clear
View Answer play_arrow
question_answer 36) An object is placed at a distance equal to focal length of convex mirror. If the focal length of the mirror be\[f\], then the distance of the image from the pole of the mirror is:
A)
less than\[f\]
done
clear
B)
equal to\[f\]
done
clear
C)
more than\[f\]
done
clear
D)
infinity
done
clear
View Answer play_arrow
question_answer 37) Ability of the eye to see objects at all distances is called:
A)
binocular vision
done
clear
B)
myopia
done
clear
C)
hypermetropia
done
clear
D)
accommodation
done
clear
View Answer play_arrow
question_answer 38) To increase magnifying power of telescope, we should increase:
A)
the focal length of the objective
done
clear
B)
the focal length of the eyepiece
done
clear
C)
aperture of the objective
done
clear
D)
aperture of the eyepiece
done
clear
View Answer play_arrow
question_answer 39) The magnitude of saturation photoelectric current depends upon:
A)
frequency
done
clear
B)
intensity
done
clear
C)
work function
done
clear
D)
stopping potential
done
clear
View Answer play_arrow
question_answer 40) Which of the following is most unstable?
A)
Electron
done
clear
B)
Proton
done
clear
C)
Neutron
done
clear
D)
\[\alpha -\]particle
done
clear
View Answer play_arrow
question_answer 41) After \[2\,\,h\,\,\frac{1}{16}th\] of initial amount of a certain radioactive isotope remains undecided. The half-life of the isotope is:
A)
\[15\min \]
done
clear
B)
\[30\min \]
done
clear
C)
\[45\min \]
done
clear
D)
\[60\min \]
done
clear
View Answer play_arrow
question_answer 42) \[10\,\,g\]of ice at \[{{0}^{o}}C\] is mixed with \[100\,\,g\] of water at \[{{50}^{o}}C\]. What is the resultant temperature of mixture?
A)
\[{{31.2}^{o}}C\]
done
clear
B)
\[{{32.8}^{o}}C\]
done
clear
C)
\[{{36.7}^{o}}C\]
done
clear
D)
\[{{38.2}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 43) An atomiser is based on the application of:
A)
Torricellis theorem
done
clear
B)
Bernoullis theorem
done
clear
C)
Archimedes principle
done
clear
D)
principle of continuity
done
clear
View Answer play_arrow
question_answer 44) Pascal-second has the dimensions of:
A)
force
done
clear
B)
energy
done
clear
C)
pressure
done
clear
D)
coefficient of viscosity
done
clear
View Answer play_arrow
question_answer 45) Consider the following equation of Bernoullis theorem \[P+\frac{1}{2}\rho {{v}^{2}}+\rho gh=K\](constant) The dimensions of \[K/P\] are same as that of which of the following?
A)
Thrust
done
clear
B)
Pressure
done
clear
C)
Angle
done
clear
D)
Viscosity
done
clear
View Answer play_arrow
question_answer 46) What effect occurs on the frequency of a pendulum if it is taken from the earths surface to deep into a mine?
A)
Increases
done
clear
B)
Decreases
done
clear
C)
First increases then decreases
done
clear
D)
No effect
done
clear
View Answer play_arrow
question_answer 47) The crystal of diamond shines due to:
A)
high density
done
clear
B)
total internal reflection
done
clear
C)
crystal lattice
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 48) The volume of a cube in \[{{m}^{3}}\] is equal to the surface area of the cube in\[{{m}^{2}}\]. The volume of the cube is:
A)
\[64{{m}^{3}}\]
done
clear
B)
\[216{{m}^{3}}\]
done
clear
C)
\[512{{m}^{3}}\]
done
clear
D)
\[196{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 49) An ice block contains a glass ball. When the ice melts within the water containing vessel, the level of water:
A)
rises
done
clear
B)
falls
done
clear
C)
unchanged
done
clear
D)
first rises and then falls
done
clear
View Answer play_arrow
question_answer 50) A beaker is completely filled with water at\[{{4}^{o}}C.\] It will overflow if:
A)
heated above\[{{4}^{o}}C\]
done
clear
B)
cooled below\[{{4}^{o}}C\]
done
clear
C)
both heated and cooled above and below\[{{4}^{o}}C\]respectively
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 51) The mass of a photon with wavelength\[3.6\overset{\text{o}}{\mathop{\text{A}}}\,\]is:
A)
\[6.135\times {{10}^{-29}}kg\]
done
clear
B)
\[3.60\times {{10}^{-29}}kg\]
done
clear
C)
\[6.135\times {{10}^{-33}}kg\]
done
clear
D)
\[3.60\times {{10}^{-27}}kg\]
done
clear
View Answer play_arrow
question_answer 52) An electron with values 4, 2, -2 and +1/2 for the set of four quantum numbers \[n,\,\,l,\,\,{{m}_{l}}\] and\[s\]respectively, belongs to:
A)
\[4s\]orbital
done
clear
B)
\[4p\]orbital
done
clear
C)
\[4d\]orbital
done
clear
D)
\[4f\]orbital
done
clear
View Answer play_arrow
question_answer 53) The bond order of\[O_{2}^{+},\,\,{{O}_{2}},\,\,O_{2}^{2-}\]and\[O_{2}^{-}\]varies in the order:
A)
\[O_{2}^{+}>{{O}_{2}}>O_{2}^{2-}>O_{2}^{-}\]
done
clear
B)
\[O_{2}^{2-}>O_{2}^{-}>{{O}_{2}}>O_{2}^{+}\]
done
clear
C)
\[O_{2}^{+}>{{O}_{2}}>O_{2}^{-}>O_{2}^{2-}\]
done
clear
D)
\[{{O}_{2}}>O_{2}^{+}>O_{2}^{-}>O_{2}^{2-}\]
done
clear
View Answer play_arrow
question_answer 54) Molecular orbital electronic configuration for\[X\]anion is: \[KK*{{(\sigma 2s)}^{2}}{{(\sigma *2s)}^{2}}{{(\pi 2{{p}_{x}})}^{2}}{{(\pi 2{{p}_{y}})}^{2}}{{(\sigma 2{{p}_{z}})}^{2}}\]\[{{(\pi *2{{p}_{x}})}^{1}}\]. The anion \[X\] is:
A)
\[N_{2}^{-}\]
done
clear
B)
\[O_{2}^{-}\]
done
clear
C)
\[N_{2}^{2-}\]
done
clear
D)
\[O_{2}^{2-}\]
done
clear
View Answer play_arrow
question_answer 55) Which of the following statements is incorrect?
A)
Pure aluminium oxide is obtained by heating aluminium hydroxide.
done
clear
B)
Cryolite lowers down the melting temperature of bauxite in the electrolytic cell for extraction of aluminium.
done
clear
C)
Carbonate ores are converted into oxides by roasting ore in air.
done
clear
D)
Mercury cannot be produced by roasting the ore cinnabar in air.
done
clear
View Answer play_arrow
question_answer 56) The relative Lewis acid character of boron trihalides is in the order:
A)
\[B{{I}_{3}}>BB{{r}_{3}}>B{{F}_{3}}>BC{{l}_{3}}\]
done
clear
B)
\[B{{I}_{3}}>BB{{r}_{3}}>BC{{l}_{3}}>B{{F}_{3}}\]
done
clear
C)
\[B{{F}_{3}}>BC{{l}_{3}}>BB{{r}_{3}}>B{{I}_{3}}\]
done
clear
D)
\[BC{{l}_{3}}>B{{F}_{3}}>B{{I}_{3}}>BB{{r}_{3}}\]
done
clear
View Answer play_arrow
question_answer 57) Which of the following metals is most reactive towards water?
A)
\[Na\]
done
clear
B)
\[K\]
done
clear
C)
\[Rb\]
done
clear
D)
\[Cs\]
done
clear
View Answer play_arrow
question_answer 58) Which of the following molecules has pentagonal bipyramidal shape?
A)
\[P{{F}_{5}}\]
done
clear
B)
\[S{{F}_{6}}\]
done
clear
C)
\[Xe{{F}_{6}}\]
done
clear
D)
\[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 59) The acidity of hydrides of \[O,\,\,S,\,\,Se,\,\,Te\] varies in the order:
A)
\[{{H}_{2}}O>{{H}_{2}}S>{{H}_{2}}Se>{{H}_{2}}Te\]
done
clear
B)
\[{{H}_{2}}O<{{H}_{2}}S<{{H}_{2}}Se<{{H}_{2}}Te\]
done
clear
C)
\[{{H}_{2}}S>{{H}_{2}}O>{{H}_{2}}Se>{{H}_{2}}Te\]
done
clear
D)
\[{{H}_{2}}Se>{{H}_{2}}S>{{H}_{2}}O>{{H}_{2}}Te\]
done
clear
View Answer play_arrow
question_answer 60) The stability of complexes of\[C{{u}^{2+}},\,\,N{{i}^{2+}},\,\,C{{o}^{2+}}\]and \[F{{e}^{2+}}\] varies in the order:
A)
\[C{{u}^{2+}}>N{{i}^{2+}}>C{{o}^{2+}}>F{{e}^{2+}}\]
done
clear
B)
\[C{{u}^{2+}}>F{{e}^{2+}}>N{{i}^{2+}}>C{{o}^{2+}}\]
done
clear
C)
\[N{{i}^{2+}}>C{{o}^{2+}}>F{{e}^{2+}}>C{{u}^{2+}}\]
done
clear
D)
\[C{{u}^{2+}}>N{{i}^{2+}}>C{{o}^{2+}}>F{{e}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 61) Which of the following compounds is generally used for hydrogenation of alkenes?
A)
\[Ni{{(CO)}_{4}}\]
done
clear
B)
\[{{[{{({{C}_{6}}{{H}_{5}})}_{3}}P]}_{3}}RhCl\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}Al\]
done
clear
D)
\[{{({{C}_{5}}{{H}_{5}})}_{2}}Fe\]
done
clear
View Answer play_arrow
question_answer 62) The example of co-ordination isomerism is;
A)
\[[Co{{(N{{H}_{3}})}_{6}}][Cr{{(CN)}_{6}}]\]and\[[Cr{{(N{{H}_{3}})}_{6}}][Co{{(CN)}_{6}}]\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{5}}Br]S{{O}_{4}}\]and\[[Co{{(N{{H}_{3}})}_{5}}S{{O}_{4}}]Br\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{5}}N{{O}_{3}}]S{{O}_{4}}\]and\[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]B{{r}_{2}}\]
done
clear
D)
\[[Pt{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]B{{r}_{2}}\]and\[[Pt{{(N{{H}_{3}})}_{4}}B{{r}_{2}}]C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 63) Cow milk is an example of natural emulsion stabilized by:
A)
detergent
done
clear
B)
fat
done
clear
C)
casein
done
clear
D)
urea
done
clear
View Answer play_arrow
question_answer 64) The molal elevation constant for water is\[0.52\]. What will be the boiling point of 2 molar sucrose solution at \[1atm\] pressure? (Assume B.P. of pure water as \[{{100}^{o}}C\])
A)
\[{{101.04}^{o}}C\]
done
clear
B)
\[{{100.26}^{o}}C\]
done
clear
C)
\[{{100.52}^{o}}C\]
done
clear
D)
\[{{99.74}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 65) If at \[{{25}^{o}}C\] the ionization constant of acetic acid is \[2\times {{10}^{-5}}\], the hydrolysis constant of sodium acetate will be:
A)
\[5\times {{10}^{-8}}\]
done
clear
B)
\[5\times {{10}^{-9}}\]
done
clear
C)
\[5\times {{10}^{-10}}\]
done
clear
D)
\[4\times {{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 66) The solubility product of \[A{{g}_{2}}CrO\] in water a\[298K\]is\[3.2\times {{10}^{-11}}\]. What will be the concentration of \[CrO_{4}^{2-}\] ions in the saturate solution of\[A{{g}_{2}}Cr{{O}_{4}}\]?
A)
\[2\times {{10}^{-4}}M\]
done
clear
B)
\[5.7\times {{10}^{-5}}M\]
done
clear
C)
\[5.7\times {{10}^{-6}}M\]
done
clear
D)
\[3.2\times {{10}^{-11}}\]
done
clear
View Answer play_arrow
question_answer 67) The \[p{{K}_{b}}\] value of \[N{{H}_{3}}\] is 5. Calculate the \[pH\] of the buffer solution, \[1\,\,L\] of which contains\[0.01\,\,M\,\,N{{H}_{4}}Cl\]and\[0.10\,\,M\,\,N{{H}_{4}}OH\]:
A)
4
done
clear
B)
6
done
clear
C)
8
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 68)
Calculate the entropy change for\[C{{H}_{4}}(g)+{{H}_{2}}O(g)\xrightarrow{{}}3{{H}_{2}}(g)+CO(g)\],using the following data: Substance \[C{{H}_{4}}(g)\] \[{{H}_{2}}O(g)\] \[{{H}_{2}}(g)\] \[CO(g)\] \[\mathbf{S{}^\circ /J}{{\mathbf{K}}^{\mathbf{-1}}}\mathbf{mo}{{\mathbf{l}}^{\mathbf{-1}}}\] \[186.2\] \[188.7\] \[130.6\] \[197.6\]
The entropy change is:
A)
\[-46J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
B)
\[+46J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
C)
\[-214.5J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
D)
\[+214.5J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 69) The type of hybridization in \[Xe{{F}_{4}}\] is:
A)
\[ds{{p}^{2}}\]
done
clear
B)
\[s{{p}^{3}}d\]
done
clear
C)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
D)
\[s{{p}^{3}}{{d}^{3}}\]
done
clear
View Answer play_arrow
question_answer 70) Given the electrode potentials\[F{{e}^{3+}}+{{e}^{-}}\xrightarrow{{}}F{{e}^{2+}},\,\,E{}^\circ =0.771\,\,volt\]\[{{I}_{2}}+2{{e}^{-}}\xrightarrow{{}}2{{I}^{-}},\,\,E{}^\circ =0.536\,\,volt\] \[E_{cell}^{\text{o}}\] for the cell reaction\[2F{{e}^{3+}}+2{{I}^{-}}\xrightarrow{{}}2F{{e}^{2+}}+{{I}_{2}},\]is:
A)
\[1.006V\]
done
clear
B)
\[0.503V\]
done
clear
C)
\[0.235V\]
done
clear
D)
\[-0.235V\]
done
clear
View Answer play_arrow
question_answer 71) In the general nuclear reaction\[_{Z}^{A}X+_{2}^{4}He\to _{Z+1}^{Z+3}Y+D\] The product \[D\] is:
A)
\[\alpha -\]particle
done
clear
B)
\[\beta -\]particle
done
clear
C)
\[\gamma -\]particle
done
clear
D)
proton
done
clear
View Answer play_arrow
question_answer 72) Lasagnes test is not used for the detection of:
A)
carbon
done
clear
B)
halogens
done
clear
C)
nitrogen
done
clear
D)
sulphur
done
clear
View Answer play_arrow
question_answer 73) The number of isomeric alkenes with molecular formula \[{{C}_{6}}{{H}_{12}}\] are:
A)
8
done
clear
B)
10
done
clear
C)
11
done
clear
D)
13
done
clear
View Answer play_arrow
question_answer 74) Which of the following alkenes will yield \[2-\]butanone on ozonolysis followed by the reaction with\[Zn/{{H}_{2}}O\]?
A)
2-methyl-2-hexene
done
clear
B)
2-methyl-1-hexene
done
clear
C)
3, 4-dimethyl-3-hexene
done
clear
D)
2, 3-dimethyl-3-hexene
done
clear
View Answer play_arrow
question_answer 75) A bromoalkane \[X\] reacts with magnesium in dry ether to form compound\[Y\]. The reaction of \[Y\] with methanal followed by hydrolysis yield an alcohol having molecular formula\[{{C}_{4}}{{H}_{10}}O\]. The compound \[X\] is:
A)
bromo ethane
done
clear
B)
bromo methane
done
clear
C)
1-bromo propane
done
clear
D)
2-bromo propane
done
clear
View Answer play_arrow
question_answer 76) Which of the following compounds will not exhibit \[cis-trans\] isomerism?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 77) Which of the following molecules is achiral?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 78) Which of the following compounds will be most reactive towards nucleophilic addition reaction?
A)
\[C{{H}_{3}}COC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}CHO\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-CO-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following compounds is the strongest acid?
A)
\[HCOOH\]
done
clear
B)
\[C{{H}_{3}}COOH\]
done
clear
C)
\[C{{l}_{2}}CHCOOH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}COOH\]
done
clear
View Answer play_arrow
question_answer 80)
In the reaction sequence
A)
benzonitrile
done
clear
B)
benzaldehyde
done
clear
C)
benzoicacid
done
clear
D)
benzyldmine
done
clear
View Answer play_arrow
question_answer 81) The basicity of compounds I, II, III and IV\[\underset{I}{\mathop{C{{H}_{3}}N{{H}_{2}}}}\,,\underset{II}{\mathop{{{(C{{H}_{3}})}_{2}}NH}}\,,\,\,\underset{III}{\mathop{{{(C{{H}_{3}})}_{3}}N}}\,,\]\[\underset{IV}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{2}}N{{H}_{2}}}}\,\]varies in the order:
A)
\[I>II>III>IV\]
done
clear
B)
\[II>I>III>IV\]
done
clear
C)
\[III>I>II>IV\]
done
clear
D)
\[IV>I>II>III\]
done
clear
View Answer play_arrow
question_answer 82) Diethyl oxalate is used for distinguishing primary, secondary and tertiary:
A)
alcohols
done
clear
B)
amines
done
clear
C)
alkylhalides
done
clear
D)
hydrogens in hydrocarbons
done
clear
View Answer play_arrow
question_answer 83) The brown haze of photochemical smog is largely attributable to:
A)
\[NO\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[C{{H}_{3}}\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,ON{{O}_{2}}\]
done
clear
D)
\[C{{H}_{2}}=CHCH=O\]
done
clear
View Answer play_arrow
question_answer 84) The water pollutants mainly responsible for eutrophication are:
A)
\[Cd,\,\,Pb\]and \[Hg\] present in industrial waste
done
clear
B)
heavy metals present in mining waste
done
clear
C)
detergents and fertilizers containing phosphate anion
done
clear
D)
polychlorinated biphenyls
done
clear
View Answer play_arrow
question_answer 85) Which of the following is a thermosetting polymer?
A)
Polyethene
done
clear
B)
Bakelite
done
clear
C)
Terylene
done
clear
D)
Polystyrene
done
clear
View Answer play_arrow
question_answer 86) Which of the following is an example of conjugated protein?
A)
Albumin
done
clear
B)
Globulin
done
clear
C)
Glutelin
done
clear
D)
Glycoprotein
done
clear
View Answer play_arrow
question_answer 87) If one strand of DNA has the sequence \[ATCGTATG\], the sequence in the complementary strand would be:
A)
\[TAGCTTAC\]
done
clear
B)
\[TCACATAC\]
done
clear
C)
\[TAGCATAC\]
done
clear
D)
\[TACGATAC\]
done
clear
View Answer play_arrow
question_answer 88) Fluorescein is an example of:
A)
azodyes
done
clear
B)
phthalein dyes
done
clear
C)
triphenyl methane dyes
done
clear
D)
nitro dyes
done
clear
View Answer play_arrow
question_answer 89) Amphetamine is used as:
A)
anaesthetic
done
clear
B)
antidepressant
done
clear
C)
antimalarial
done
clear
D)
analgesic
done
clear
View Answer play_arrow
question_answer 90) Which of the following compounds is used as broad spectrum antibiotics?
A)
Ampicillin
done
clear
B)
Penicillin G
done
clear
C)
Penicillin K
done
clear
D)
Tetracycline
done
clear
View Answer play_arrow
question_answer 91) The weight in gram of 1 curie of radioactive element \[^{200}X\] having \[a\,\,{{t}_{1/2}}\] of \[69.3\min \] is:
A)
\[3.7\times {{10}^{-8}}g\]
done
clear
B)
\[7.4\times {{10}^{-8}}g\]
done
clear
C)
\[3.7\times {{10}^{-8}}g\]
done
clear
D)
\[200g\]
done
clear
View Answer play_arrow
question_answer 92) Gold number is minimum in case of:
A)
gum arable
done
clear
B)
starch
done
clear
C)
egg albumin
done
clear
D)
gelatin
done
clear
View Answer play_arrow
question_answer 93) The standard reduction potential of \[L{{i}^{+}}/Li\]\[B{{a}^{2+}}/Ba,\,\,N{{a}^{+}}/Na\] and \[M{{g}^{2+}}/Mg\] are\[-3.05\],\[-2.73\], \[-2.71\]and \[-2.37\] volt respectively, Which one of the following is strongest oxidising agent?
A)
\[B{{a}^{2+}}\]
done
clear
B)
\[M{{g}^{2+}}\]
done
clear
C)
\[N{{a}^{+}}\]
done
clear
D)
\[L{{i}^{+}}\]
done
clear
View Answer play_arrow
question_answer 94) Carbon is in the lowest oxidation state in:
A)
\[C{{O}_{2}}\]
done
clear
B)
\[C{{F}_{4}}\]
done
clear
C)
\[CC{{l}_{4}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 95) Oxidation state of nitrogen is correctly given for: Compound Oxidation state
A)
\[M{{g}_{3}}{{N}_{2}}\] \[-3\]
done
clear
B)
\[N{{H}_{2}}OH\] \[+1\]
done
clear
C)
\[{{({{N}_{2}}{{H}_{5}})}_{2}}S{{O}_{4}}\] \[+2\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}\] \[0\]
done
clear
View Answer play_arrow
question_answer 96) The solubility product of \[AgCl\] is \[4.0\times {{10}^{-10}}\] at\[298K\]. The solubility of \[AgCl\] in \[0.04\,\,M\,\,CaC{{l}_{2}}\] will be:
A)
\[5.0\times {{10}^{-9}}M\]
done
clear
B)
\[2.0\times {{10}^{-5}}M\]
done
clear
C)
\[2.2\times {{10}^{-4}}M\]
done
clear
D)
\[1.0\times {{10}^{-4}}M\]
done
clear
View Answer play_arrow
question_answer 97) The standard free energy change \[(\Delta G{}^\circ )\] is related to equilibrium constant \[(K)\] as:
A)
\[\Delta G{}^\circ =2.303RT\log k\]
done
clear
B)
\[\Delta G{}^\circ =RT\log k\]
done
clear
C)
\[\Delta G{}^\circ =2.303RT\log k\]
done
clear
D)
\[\Delta G{}^\circ =-2.303RT\,\,in\,\,k\]
done
clear
View Answer play_arrow
question_answer 98) For reversible process, the value of AS is gives by the expression:
A)
\[{{q}_{(rev)}}\times T\]
done
clear
B)
\[{{q}_{(rev)}}\div T\]
done
clear
C)
\[\Delta H/\Delta T\]
done
clear
D)
\[T\div {{q}_{(rev)}}\]
done
clear
View Answer play_arrow
question_answer 99)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 100) Tincture of iodine is:
A)
\[CH{{I}_{3}}\]in alcohol
done
clear
B)
\[{{I}_{2}}\]alcohol
done
clear
C)
\[{{I}_{2}}\]in\[KI\]
done
clear
D)
\[CH{{I}_{3}}\]in\[KI\]
done
clear
View Answer play_arrow
question_answer 101) The protein toxin producing bacteria, which used to control biological pest is:
A)
E. coli
done
clear
B)
Agrobacterium
done
clear
C)
Mycobacterium sp.
done
clear
D)
B. thuringiensis
done
clear
View Answer play_arrow
question_answer 102) Bacteria with single flagella at one end is called:
A)
monotrichous
done
clear
B)
lophotrichous
done
clear
C)
amphitrichous
done
clear
D)
peritrichpus
done
clear
View Answer play_arrow
question_answer 103) Which group is evolutionary modem?
A)
Gymnosperms
done
clear
B)
Grasses
done
clear
C)
Pteridophytes
done
clear
D)
Algae
done
clear
View Answer play_arrow
question_answer 104) Most stable ecosystem is:
A)
desert
done
clear
B)
marine
done
clear
C)
mountain
done
clear
D)
forest
done
clear
View Answer play_arrow
question_answer 105) Stratification occurs in:
A)
desert
done
clear
B)
tropical forest
done
clear
C)
deciduous forest
done
clear
D)
tundra
done
clear
View Answer play_arrow
question_answer 106) Ecological pyramids were discovered by:
A)
Eiton
done
clear
B)
Odum
done
clear
C)
Reiter
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 107) The cyrstal of lead zirconate is a key component of:
A)
electroencephalography
done
clear
B)
electrocardiography
done
clear
C)
magneto encephalography
done
clear
D)
sonography
done
clear
View Answer play_arrow
question_answer 108) Which one of the following is a matching pair of a certain body feature and its value/count in a normal human adult?
A)
Urea \[5-10mg/100ml\] of blood.
done
clear
B)
Blood sugar (fasting)\[-70-100\text{ }mg/100ml\]
done
clear
C)
Total blood volume\[-5-6\]
done
clear
D)
ESR in Wintrobe method \[-9-15mm\] in males and \[20-34mm\] in females
done
clear
View Answer play_arrow
question_answer 109) Which one of the following is a matching pair?
A)
Lubb-Sharp closure of AV valves at the beginning of ventricular systole
done
clear
B)
Dup-Sudden opening of semilunar valves at the beginning of ventricular diastole
done
clear
C)
Pulsation of the radial artery valves in the blood vessels
done
clear
D)
Initiation of the heart beat Purkinje fibres
done
clear
View Answer play_arrow
question_answer 110) July 11 is observed as:
A)
World Population Day
done
clear
B)
No Tobacco Day
done
clear
C)
World Environment Day
done
clear
D)
World Health Day
done
clear
View Answer play_arrow
question_answer 111) Biological Oxygen Demand (BOD) is a measure of:
A)
industrial wastes poured into water bodies
done
clear
B)
extent to which water is polluted with organic compound
done
clear
C)
amount of carbon monoxide inseparably combined with haemoglobin
done
clear
D)
amount of oxygen needed by green plants during night
done
clear
View Answer play_arrow
question_answer 112) Photorespiration in \[{{C}_{3}}\] plants starts from :
A)
phosphoglycerate
done
clear
B)
phosphoglycolate
done
clear
C)
glycerate
done
clear
D)
glycine
done
clear
View Answer play_arrow
question_answer 113) Continued consumption of a diet rich in butter, red meat and eggs for a long period may lead to:
A)
vitamin-A toxicity
done
clear
B)
kidney stones
done
clear
C)
hypercholesterolemia
done
clear
D)
urine laden with ketone bodies
done
clear
View Answer play_arrow
question_answer 114) What is true about \[t-\]RNA?
A)
It binds with an amino acid at it 3 end
done
clear
B)
It has five double stranded regions
done
clear
C)
It has a codon at one end which recognizes the anticodon on messenger RNA
done
clear
D)
It looks like clover leaf in the three dimensional structure
done
clear
View Answer play_arrow
question_answer 115) Photochemical smog formed in congested metropolitan cities mainly consists of:
A)
ozone, peroxyacetyl nitrate and\[N{{O}_{x}}\]
done
clear
B)
smoke, peroxyacetyl nitrate and\[S{{O}_{2}}\]
done
clear
C)
hydrocarbons, \[S{{O}_{2}}\] and\[C{{O}_{2}}\]
done
clear
D)
hydrocarbons, ozone and\[S{{O}_{x}}\]
done
clear
View Answer play_arrow
question_answer 116) Natural parthenogenesis is found in:
A)
housefly
done
clear
B)
honeybee
done
clear
C)
Drosophila
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 117) Mammals excrete:
A)
urea
done
clear
B)
uric acid
done
clear
C)
ammonia
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 118) Vestigeal organ in human being is :
A)
incisor
done
clear
B)
canines
done
clear
C)
molar
done
clear
D)
premolar
done
clear
View Answer play_arrow
question_answer 119) The connection between two cells is :
A)
plasmadesmata
done
clear
B)
microfilaments
done
clear
C)
sieve tube
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 120) Natural pearl is:
A)
a mollusc
done
clear
B)
an annelid
done
clear
C)
an arthropod
done
clear
D)
an echinodermate
done
clear
View Answer play_arrow
question_answer 121) Miller and Urey performed an experiment to prove the origin of life. They took gases \[N{{H}_{3}}\] and \[{{H}_{2}}\] along with:
A)
\[{{N}_{2}}\]and\[{{H}_{2}}O\]
done
clear
B)
\[{{H}_{2}}O\]and\[C{{H}_{4}}\]
done
clear
C)
\[C{{H}_{4}}\]and\[{{N}_{2}}\]
done
clear
D)
\[C{{O}_{2}}\]and\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 122) Spermatogenesis is induced by:
A)
FSH
done
clear
B)
ACTH
done
clear
C)
ICSH
done
clear
D)
ATH
done
clear
View Answer play_arrow
question_answer 123) Blood carries \[C{{O}_{2}}\] mainly in which form?
A)
\[Hb.C{{O}_{2}}\]
done
clear
B)
\[NaHC{{O}_{3}}\]
done
clear
C)
Carbonic acid
done
clear
D)
\[Hb.C{{O}_{2}}\]and carbon monoxide
done
clear
View Answer play_arrow
question_answer 124) During oxidative phosphorylation, the net gain of ATP is:
A)
40
done
clear
B)
38
done
clear
C)
34
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 125) Which of the following pairs is correct?
A)
Annelida-polychaeta-leech
done
clear
B)
Arthropoda-crustacea-cockroach
done
clear
C)
Mollusca-cephalopoda-Octopus
done
clear
D)
Protozoa-Hydra
done
clear
View Answer play_arrow
question_answer 126) In almost all Indian metropolitan cities like Delhi, the major atmospheric pollutant(s) is/are:
A)
suspended paniculate matter (SPM)
done
clear
B)
oxides of sulphur
done
clear
C)
carbon dioxide and carbon monoxide
done
clear
D)
oxides of nitrogen
done
clear
View Answer play_arrow
question_answer 127) Restriction enzymes:
A)
are endonucleases which cleave DNA at specific sites
done
clear
B)
make DNA complementary to an existing DNA or RNA
done
clear
C)
cut or join DNA fragments
done
clear
D)
are required in vectorless direct gene transfer
done
clear
View Answer play_arrow
question_answer 128) The phase of menstrual cycle in humans that lasts for 7-8 days, is:
A)
follicular phase
done
clear
B)
ovulatory phase
done
clear
C)
luteal phase
done
clear
D)
menstruation
done
clear
View Answer play_arrow
question_answer 129) The source of somatostatin is same as that of:
A)
thyroxine and calcitonin
done
clear
B)
insulin and glucagon
done
clear
C)
somatotropin and prolactin
done
clear
D)
vasopressin and oxytocin
done
clear
View Answer play_arrow
question_answer 130) Reserpine is obtained from:
A)
Asafoetida
done
clear
B)
Rauwolffia serpentine
done
clear
C)
Curcuma longa
done
clear
D)
Papaver somniferum
done
clear
View Answer play_arrow
question_answer 131) The coiling of tendril around some base in response to touch is called:
A)
hydrotaxis
done
clear
B)
chemotaxis
done
clear
C)
thigmotropism
done
clear
D)
geotaxis
done
clear
View Answer play_arrow
question_answer 132) A characteristic feature of ovary of Brassica campestris is:
A)
presence of replum
done
clear
B)
axile placentation
done
clear
C)
epigynous
done
clear
D)
multilocular nature
done
clear
View Answer play_arrow
question_answer 133) One of the characteristic of Hydra is:
A)
predation
done
clear
B)
metamerism
done
clear
C)
hibernation
done
clear
D)
mimicry
done
clear
View Answer play_arrow
question_answer 134) Primary acceptor of \[C{{O}_{2}}\] in photosynthesis is:
A)
phosphoric acid
done
clear
B)
ribulose phosphate
done
clear
C)
glucose
done
clear
D)
ribulose 1, 5 biphosphate
done
clear
View Answer play_arrow
question_answer 135) Which of the following statement is correct?
A)
In Cycas, megasporophyll produce pollen grains
done
clear
B)
In Agaricus, gills produce basidiospores
done
clear
C)
In Aspergillus, fruiting body is perithecium
done
clear
D)
In Funaria, capsule represents gameto- phytic generation
done
clear
View Answer play_arrow
question_answer 136) Which of the following is a disaccharide?
A)
Glucose
done
clear
B)
Fructose
done
clear
C)
Sucrose
done
clear
D)
Galactose
done
clear
View Answer play_arrow
question_answer 137) Periplaneta has no respiratory pigment in its blood because:
A)
air is conducted directly to the body tissues
done
clear
B)
it has haemocoelom
done
clear
C)
it has anaerobic respiration
done
clear
D)
it lacks blood cell in blood
done
clear
View Answer play_arrow
question_answer 138) Which is correct for meiotic metaphase-I?
A)
Bivalents are arranged at equator
done
clear
B)
Univalents are arranged at equator
done
clear
C)
Non-homologous chromosomes forms pair
done
clear
D)
Spindle fibres are attached at chromomere
done
clear
View Answer play_arrow
question_answer 139) In the following how the sap wood is converted into heart wood?
A)
By degeneration of protoplast of living cells
done
clear
B)
Tyiosis formation
done
clear
C)
By deposition of resins, oil, gums etc.
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 140) Exception of MendePs law is:
A)
independent assortment
done
clear
B)
linkage
done
clear
C)
purity of gametes
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 141) A type of life cycle in which plasmogamy, karyogamy, haplodization takes place but not at specific place in life cycle of an organism is called as:
A)
parasexuality
done
clear
B)
heterozygosity
done
clear
C)
homozygosity
done
clear
D)
asexuality
done
clear
View Answer play_arrow
question_answer 142) Branched, aseptate, coenocytic mycelium present in:
A)
Aspergillus
done
clear
B)
Albugo
done
clear
C)
Penicillium
done
clear
D)
Erysiphe
done
clear
View Answer play_arrow
question_answer 143) In cockroach vision is due to:
A)
one compound eye
done
clear
B)
only two compound eyes
done
clear
C)
two simple eyes
done
clear
D)
two compound and two simple eyes
done
clear
View Answer play_arrow
question_answer 144) When tall and dwarf plants are crossed, from which cross 1:1 ratio is obtained?
A)
\[Tt\]and\[tt\]
done
clear
B)
\[tt\]and\[tt\]
done
clear
C)
\[Tt\times Tt\]
done
clear
D)
\[TT\]and\[Tt\]
done
clear
View Answer play_arrow
question_answer 145) The cells without nuclei are present in:
A)
vascular cambium
done
clear
B)
root hair
done
clear
C)
companion cell
done
clear
D)
members of sieve tube
done
clear
View Answer play_arrow
question_answer 146) Which of the following have sunken stomata?
A)
Nerium
done
clear
B)
Mangifera
done
clear
C)
Hydrilla
done
clear
D)
Zea mays
done
clear
View Answer play_arrow
question_answer 147) When a plasmolysed cells is placed in a hypotonic solution then water will move inside the cell. Which force causes this?
A)
DPD
done
clear
B)
OP
done
clear
C)
WP
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 148) Dormancy of seed is broken by:
A)
auxin
done
clear
B)
gibberellins
done
clear
C)
ethyelene
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 149) Viroids have:
A)
single stranded RNA not enclosed by protein coat
done
clear
B)
single stranded DNA not enclosed by protein coat
done
clear
C)
double stranded DNA enclosed by protein coat
done
clear
D)
double stranded RNA enclosed by protein coat
done
clear
View Answer play_arrow
question_answer 150) Which one of the following pairs is correctly matched?
A)
Rhizobium - Parasite in the roots of leguminous plants
done
clear
B)
Mycorrhizae - Mineral uptake from soil
done
clear
C)
Yeast - Production of biogas
done
clear
D)
Myxomycetes - The diesease ringworm
done
clear
View Answer play_arrow
question_answer 151) Pollen grains are able to withstand extremes of temperature and dessication because their exine is composed of:
A)
cutin
done
clear
B)
suberin
done
clear
C)
sporopollenin
done
clear
D)
callose
done
clear
View Answer play_arrow
question_answer 152) The quiescent centre in root meristem served as a:
A)
site for storage of food which is utilized during maturation
done
clear
B)
reservoir of growth hormones
done
clear
C)
reserve for replenishment of damaged cells of the meristem
done
clear
D)
region for absorption of water
done
clear
View Answer play_arrow
question_answer 153) Azolla is used as a bio fertilizer because it:
A)
multiplies very fast to produce massive biomass
done
clear
B)
has association of nitrogen-fixing Rhizobium
done
clear
C)
has association of nitrogen-fixing cyanobacteria
done
clear
D)
has association of Mycorrhiza
done
clear
View Answer play_arrow
question_answer 154) The plant part which consists of two generations one within the other, is:
A)
germinated pollen grain
done
clear
B)
embryo
done
clear
C)
unfertilized ovule
done
clear
D)
seed
done
clear
View Answer play_arrow
question_answer 155) Anti-viral substance is:
A)
antigen
done
clear
B)
antibody
done
clear
C)
interferon
done
clear
D)
antibiotic
done
clear
View Answer play_arrow
question_answer 156) The chemicals which are produced by host plants due to infection as a defence reaction to pathogen, are called:
A)
phytotoxin
done
clear
B)
toxin
done
clear
C)
phytotron
done
clear
D)
phytoalexins
done
clear
View Answer play_arrow
question_answer 157) Translocation of organic materials in plants is explained by:
A)
active transport
done
clear
B)
transpiration pull
done
clear
C)
inhibition theory
done
clear
D)
mass flow hypothesis
done
clear
View Answer play_arrow
question_answer 158) Cycas stem shows:
A)
porous wood
done
clear
B)
monoxylic wood
done
clear
C)
pycnoxylic wood
done
clear
D)
ring porous wood
done
clear
View Answer play_arrow
question_answer 159) The desert plants in order to tolerate water stress show:
A)
sunken stomata
done
clear
B)
reduced leaves
done
clear
C)
well developed root system
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 160) Meiosis in Dryopteris takes place during:
A)
spore formation
done
clear
B)
gametic formation
done
clear
C)
spore germination
done
clear
D)
zygote formation
done
clear
View Answer play_arrow
question_answer 161) Photosynthetic pigments in chloroplast are embeded in the membrane of:
A)
photoglobin
done
clear
B)
matrix
done
clear
C)
thylakoid
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 162) Aerenchyma is found in:
A)
parenchyma
done
clear
B)
xylem
done
clear
C)
phloem
done
clear
D)
sclerenchyma
done
clear
View Answer play_arrow
question_answer 163) The process through which the amount of DNA, RNA and protein can be known at a time is called:
A)
autoradiography
done
clear
B)
tissue culture
done
clear
C)
cellular fractioning
done
clear
D)
phase contrast microscopy
done
clear
View Answer play_arrow
question_answer 164) Sponges capture food with the help of:
A)
pinacocytes
done
clear
B)
choanocytes
done
clear
C)
trophocytes
done
clear
D)
theocytes
done
clear
View Answer play_arrow
question_answer 165) In frog, gastrulation process involves:
A)
epiboly
done
clear
B)
emboly
done
clear
C)
invagination
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 166) To yield more milk, cow is injected with:
A)
sorbitol
done
clear
B)
prolactin
done
clear
C)
gonadotrophs
done
clear
D)
stelbesterol
done
clear
View Answer play_arrow
question_answer 167) Balbiani rings are found in :
A)
polysomes
done
clear
B)
polytene chromosomes
done
clear
C)
autosomes
done
clear
D)
non-sense chromosomes
done
clear
View Answer play_arrow
question_answer 168) One of the nucleotide of DNA:
A)
adenine
done
clear
B)
deoxyadenylic acid
done
clear
C)
adenosine
done
clear
D)
deoxyuridine phosphate
done
clear
View Answer play_arrow
question_answer 169) What is haemozine?
A)
Undigested part of blood in trophozoite of Plasmodium
done
clear
B)
Blood pigment of Anopheles
done
clear
C)
Decomposed blood in merozoites
done
clear
D)
Granules in the blood of infected person
done
clear
View Answer play_arrow
question_answer 170) Insects eggs are:
A)
microlecithal and centrolecithal
done
clear
B)
megalecithal and isolecithal
done
clear
C)
megalecithal and centrolecithal
done
clear
D)
megalecithal and telolacithal
done
clear
View Answer play_arrow
question_answer 171) Which type of rhizoids are present in Riccia?
A)
Unicellular smooth
done
clear
B)
Multicellular smooth
done
clear
C)
Unicellular smooth and tuberculated
done
clear
D)
Multicellular smooth and tuberculated
done
clear
View Answer play_arrow
question_answer 172) Which character is found only in mammals?
A)
Neck
done
clear
B)
Diaphragm
done
clear
C)
Optic lobes of brain
done
clear
D)
Tail
done
clear
View Answer play_arrow
question_answer 173) Which type of vascular bundles are found in monocot stem?
A)
Collateral, open, endarch
done
clear
B)
Radial, open, diarch
done
clear
C)
Radial, open, mesarch
done
clear
D)
Collateral, closed, endarch
done
clear
View Answer play_arrow
question_answer 174) In rhizome of Pteridium, stele which is composed of two or more than two concentric rings of vascular bundles is called:
A)
polycyclic
done
clear
B)
siphonostele
done
clear
C)
ectophloic siphonostele
done
clear
D)
cladosiphonostele
done
clear
View Answer play_arrow
question_answer 175) Which of the following is not the correct for gastrulation?
A)
Archenteron is formed
done
clear
B)
all germinal layers are formed
done
clear
C)
Morphogenetic movements
done
clear
D)
Some blastomeres and blastocoel degenerate
done
clear
View Answer play_arrow
question_answer 176) What happens in anterior part of Amoeba at the time of formation of pseudopodia?
A)
Plasma gel convert into plasma sol
done
clear
B)
Plasma sol convert into plasma gel
done
clear
C)
Ectoplasm convert into endoplasm
done
clear
D)
Endoplasm convert into ectoplasm
done
clear
View Answer play_arrow
question_answer 177) The function of clitellum in Pheretima is:
A)
formation of cocoon
done
clear
B)
secretion of hormone
done
clear
C)
nutrition of sperm
done
clear
D)
respiration
done
clear
View Answer play_arrow
question_answer 178) In Mirabilis a hybrid for red (RR) and white (rr) flower produces pink (Rr) flower. A plant with pink flower is crossed with white flower the expected phenotypic ratio is:
A)
red : pink : white\[(1:2:1)\]
done
clear
B)
pink : white\[(1:1)\]
done
clear
C)
red : pink\[(1:1)\]
done
clear
D)
red : white\[(3:1)\]
done
clear
View Answer play_arrow
question_answer 179) What is pollen grain?
A)
Microspore mother cell
done
clear
B)
Male gamete
done
clear
C)
Male gametophyte
done
clear
D)
Partially developed embryo
done
clear
View Answer play_arrow
question_answer 180) Conditions helpful in photorespiration are :
A)
more \[{{O}_{2}}\] and less \[C{{O}_{2}}\]
done
clear
B)
less \[{{O}_{2}}\] and more \[C{{O}_{2}}\]
done
clear
C)
more temperature and less \[{{O}_{2}}\]
done
clear
D)
more humidity and less temperature
done
clear
View Answer play_arrow
question_answer 181) Bicollateral bundles are found in:
A)
Cucurbitaceae
done
clear
B)
Malvaceae
done
clear
C)
Cruciferae
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 182) Lignification is associated with:
A)
xylem
done
clear
B)
phloem
done
clear
C)
parenchyma
done
clear
D)
chlorenchyma
done
clear
View Answer play_arrow
question_answer 183) Which of the following is the result of double fertilization?
A)
Cotyledon
done
clear
B)
Nucellus
done
clear
C)
Endosperm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 184) Pollen tube entered through micropyle in:
A)
porogamy
done
clear
B)
chalazogamy
done
clear
C)
syngamy
done
clear
D)
misogamy
done
clear
View Answer play_arrow
question_answer 185) Correct name is:
A)
Brassica indica
done
clear
B)
Mangifera Indica
done
clear
C)
SOLANUM MELONGINA
done
clear
D)
Mimosa Pudica
done
clear
View Answer play_arrow
question_answer 186) Who discovered the super bugs?
A)
H.G. Khorana
done
clear
B)
Dilip Sah
done
clear
C)
Anand Mohan Chakarwarty
done
clear
D)
Robert
done
clear
View Answer play_arrow
question_answer 187) During unfavourable conditions Amoeba reproduces through:
A)
binary fission
done
clear
B)
sporulation
done
clear
C)
multiple fission
done
clear
D)
conjugation
done
clear
View Answer play_arrow
question_answer 188) Radial symmetry is found in:
A)
frog
done
clear
B)
starfish
done
clear
C)
humans
done
clear
D)
Pheretima
done
clear
View Answer play_arrow
question_answer 189) Pseudocoel is present in:
A)
Periplaneta
done
clear
B)
Ascaris
done
clear
C)
Pheretima
done
clear
D)
Hydra
done
clear
View Answer play_arrow
question_answer 190) Metameric segmentation is the main feature of:
A)
Annelida
done
clear
B)
Echinodermata
done
clear
C)
Arthropoda
done
clear
D)
Coelenterata
done
clear
View Answer play_arrow
question_answer 191) Naked DNA without histones is found in:
A)
prokaryotes
done
clear
B)
eukaiyotes
done
clear
C)
protozoa
done
clear
D)
coelenterate
done
clear
View Answer play_arrow
question_answer 192) Ammonia is converted into urea in :
A)
kidney
done
clear
B)
lungs
done
clear
C)
liver
done
clear
D)
spleen
done
clear
View Answer play_arrow
question_answer 193) Which of the following maintain the Shape of cell?
A)
Osmotic pressure
done
clear
B)
Turgor pressure
done
clear
C)
Wall pressure
done
clear
D)
Osmosis
done
clear
View Answer play_arrow
question_answer 194) Vivipary is found in:
A)
Coelenterata
done
clear
B)
Protozoa
done
clear
C)
Rabbit
done
clear
D)
Pisces
done
clear
View Answer play_arrow
question_answer 195) The ultimate energy source of ecosystem is:
A)
solar energy
done
clear
B)
biomass
done
clear
C)
producer
done
clear
D)
carbohydrates
done
clear
View Answer play_arrow
question_answer 196) Lipid bilayer is present in:
A)
plasma membrane
done
clear
B)
ribosome
done
clear
C)
chromosome
done
clear
D)
nucleolus
done
clear
View Answer play_arrow
question_answer 197) Chiasmata are formed:
A)
due to crossing over of same part between homologous chromosomes
done
clear
B)
due to crossing over of same part between non-homologous chromosomes
done
clear
C)
due to duplication of homologous and non-homologous chromosomes
done
clear
D)
due to loss of some part of chromosomes
done
clear
View Answer play_arrow
question_answer 198) Food store as oil in:
A)
Chlamydomonas
done
clear
B)
Oedogonium
done
clear
C)
Vaucheria
done
clear
D)
Chara
done
clear
View Answer play_arrow
question_answer 199) Lowest number of chromosomes are found in:
A)
Haplopappus
done
clear
B)
Cyprus
done
clear
C)
Salix
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 200) Peat moss is:
A)
Funaria
done
clear
B)
ferns
done
clear
C)
algae
done
clear
D)
Sphagnum
done
clear
View Answer play_arrow
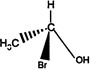
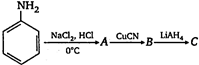 the product \[C\] is:
the product \[C\] is:
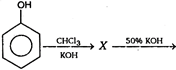 The final product of this reaction is/are:
The final product of this reaction is/are: