A) \[P{{F}_{5}}\]
B) \[S{{F}_{6}}\]
C) \[Xe{{F}_{6}}\]
D) \[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
Correct Answer: C
Solution :
In the formation of \[Xe{{F}_{6}}\] molecule, three \[5p\] electrons are promoted to \[5d\] orbitals. Now, one\[5s\], three \[5p\] and three 5d orbitals of \[Xe\] atom intermix together and form seven \[s{{p}^{3}}{{d}^{3}}\] hybrid orbitals. One \[s{{p}^{3}}{{d}^{3}}\] hybrid orbital contains one lone pair of electrons while other six are half-filled.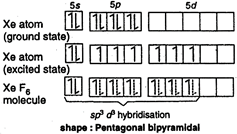 The expected geometry is pentagonal bipyramidal.
The expected geometry is pentagonal bipyramidal.
You need to login to perform this action.
You will be redirected in
3 sec
