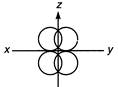
A) \[xy\]
B) \[yz\]
C) \[zx\]
D) \[{{x}^{2}}-{{y}^{2}}\]
Correct Answer: A
Solution :
\[z-z\]orbital forms Ti-bond in the diagram ie, \[{{p}_{x}}\]and\[{{p}_{y}}\]orbital use in\[s{{p}^{2}}\]hybridization.You need to login to perform this action.
You will be redirected in
3 sec
