A) \[120{}^\circ \]and 1.34\[\overset{o}{\mathop{\text{A}}}\,\]
B) \[120{}^\circ \]and 1.39\[\overset{o}{\mathop{\text{A}}}\,\]
C) \[180{}^\circ \]and 1.33\[\overset{o}{\mathop{\text{A}}}\,\]
D) \[120{}^\circ \] and 1.54\[\overset{o}{\mathop{\text{A}}}\,\]
Correct Answer: B
Solution :
Bond angle and bond length in benzene are \[120{}^\circ \]and\[1.39\overset{o}{\mathop{\text{A}}}\,\]respectively, sp2 hybridization presents in benzene. Its resonating structure is as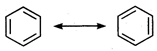
You need to login to perform this action.
You will be redirected in
3 sec
