question_answer 1) A stretched string of length\[l,\]fixed at both ends can sustain stationary waves of wavelength given by
A)
\[\lambda =\frac{{{n}^{2}}}{2l}\]
done
clear
B)
\[\lambda =\frac{{{I}^{2}}}{2n}\]
done
clear
C)
\[\lambda =\frac{2l}{n}\]
done
clear
D)
\[\lambda =2\ln \]
done
clear
View Answer play_arrow
question_answer 2) Moment of inertia of a thin circular ring of mass M and radius R about any diameter is
A)
\[M{{R}^{2}}\]
done
clear
B)
\[2M{{R}^{2}}\]
done
clear
C)
\[\frac{M{{R}^{2}}}{2}\]
done
clear
D)
\[\frac{M{{R}^{2}}}{4}\]
done
clear
View Answer play_arrow
question_answer 3) A particle moves with constant speed in circular path. During the motion its
A)
velocity is constant
done
clear
B)
acceleration is constant
done
clear
C)
radial acceleration towards the inside
done
clear
D)
radial acceleration towards the outside
done
clear
View Answer play_arrow
question_answer 4) A stone thrown upward with a speed u from the top of the tower, reaches the ground with velocity 3u. The height of the tower is
A)
\[\frac{8{{u}^{2}}}{g}\]
done
clear
B)
\[\frac{3{{u}^{2}}}{g}\]
done
clear
C)
\[\frac{4{{u}^{2}}}{g}\]
done
clear
D)
\[\frac{5{{u}^{2}}}{g}\]
done
clear
View Answer play_arrow
question_answer 5) Which one of the following is scalar?
A)
Electric potential
done
clear
B)
Momentum
done
clear
C)
Velocity
done
clear
D)
Force
done
clear
View Answer play_arrow
question_answer 6) Dimensions of torque are
A)
\[[M{{L}^{2}}{{T}^{-1}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[ML{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{-1}}{{T}^{-1}}]\]
done
clear
View Answer play_arrow
question_answer 7) If total torque of the system is zero, then the total angular momentum of the system will be constant at
A)
direction
done
clear
B)
both direction and magnitude
done
clear
C)
magnitude
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 8) The angular velocity of earth rotating about its own axis is
A)
\[\frac{2\pi }{60\,\times \,60\,\times \,2\pi }rad/s\]
done
clear
B)
\[\frac{2\pi }{60\,\times \,60}rad/s\]
done
clear
C)
\[\frac{2\pi }{60}rad/s\]
done
clear
D)
\[\frac{2\pi }{365\,\times \,24\,\times \,60\,\times \,60}rad/s\]
done
clear
View Answer play_arrow
question_answer 9) The equation of progressive wave is\[y=a\text{ }sin(200\] \[(200tx),\]where\[x\]is in metre and\[x\]is in second. The velocity of wave will be
A)
200 m/s
done
clear
B)
100 m/s
done
clear
C)
50 m/s
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 10) According to Newton's third law, which of the following statements is true?
A)
Both forces are acted upon one body
done
clear
B)
Both forces are acted upon the different bodies
done
clear
C)
Directions and magnitudes of both forces are same
done
clear
D)
Both forces have different magnitudes and opposite directions
done
clear
View Answer play_arrow
question_answer 11) If \[\overrightarrow{F}=5\widehat{i}+3\widehat{j},\,\overrightarrow{r}=2\widehat{i}-\widehat{j},\], then work done by the system will be
A)
\[-7\text{ }J\]
done
clear
B)
7 J
done
clear
C)
10 J
done
clear
D)
\[-10\text{ }J\]
done
clear
View Answer play_arrow
question_answer 12) If n is a number and u is unit of physical quantity then which of the following is correct for measurement nu?
A)
\[n\propto u\]
done
clear
B)
\[n\propto \frac{1}{u}\]
done
clear
C)
No relation between n and u
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 13) The force F is exterted one end of wire when second end is tight, the extension of wire is\[x,\]then
A)
\[F\propto x\]
done
clear
B)
\[F\propto {{x}^{2}}\]
done
clear
C)
\[F\propto {{x}^{3}}\]
done
clear
D)
\[F\propto {{x}^{4}}\]
done
clear
View Answer play_arrow
question_answer 14) If the length and radius of wire are doubled then Young's modulus of the wire will be
A)
doubled
done
clear
B)
half
done
clear
C)
constant
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 15) What is the formula of escape velocity?
A)
\[\sqrt{g{{R}_{e}}}\]
done
clear
B)
\[~2g{{R}_{e}}\]
done
clear
C)
\[\sqrt{2g{{R}_{e}}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 16) What is the true of following in an elastic collision?
A)
The kinetic energy will be conservative
done
clear
B)
The momentum will be conservative
done
clear
C)
Both kinetic energy and momentum will be conservative
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 17) If the speed of particle became twice, then which of the following quantities will be doubled?
A)
Length
done
clear
B)
Kinetic energy
done
clear
C)
Momentum
done
clear
D)
Acceleration
done
clear
View Answer play_arrow
question_answer 18) If in the lift, the body of mass 5 kg is suspended to spring balance, the lift moves downward with acceleration\[10\text{ }m/{{s}^{2}},\]then the reading of spring balance is
A)
more than 5 kg-wt
done
clear
B)
less than 5 kg-wt
done
clear
C)
5 kg-wt
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 19) The ratio of fraction of displacement of mass with which of the following quantities is constant if its motion is simple harmonic?
A)
Velocity
done
clear
B)
Acceleration
done
clear
C)
Time period
done
clear
D)
Mass
done
clear
View Answer play_arrow
question_answer 20) If the maximum acceleration of motion of a particle is\[16\text{ }m/{{s}^{2}},\]and the maximum velocity is 24 m/s, then amplitude of the particle will be
A)
36 m
done
clear
B)
20 m
done
clear
C)
16 m
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 21) Two wires A and B are made from the same material. The ratio of lengths and diameters respectively are 1:2 and 2:1. If these are stretched by a force, then the ratio of these expansion of lengths will be
A)
2 : 1
done
clear
B)
1 : 4
done
clear
C)
1 : 8
done
clear
D)
8 : 1
done
clear
View Answer play_arrow
question_answer 22) If gravitational potential at the surface of is zero, then gravitational potential at infinity will be
A)
\[{{W}_{2}}>{{W}_{1}}>{{W}_{3}}\]
done
clear
B)
\[{{W}_{2}}>{{W}_{3}}>{{W}_{1}}\]
done
clear
C)
\[{{W}_{1}}>{{W}_{2}}>{{W}_{3}}\]
done
clear
D)
\[{{W}_{1}}>{{W}_{3}}>{{W}_{2}}\]
done
clear
View Answer play_arrow
question_answer 23) Starting with the same initial conditions, an ideal gas expands from volumes\[{{V}_{1}}\]to\[{{V}_{2}}\]three different ways, the work done by the gas is \[{{W}_{1}}\] if the process is purely isothermal, \[{{W}_{2}}\] if purely isobaric and W3 if purely adiabatic, then
A)
\[{{W}_{2}}>{{W}_{1}}>{{W}_{3}}\]
done
clear
B)
\[{{W}_{2}}>{{W}_{3}}>{{W}_{1}}\]
done
clear
C)
\[{{W}_{1}}>{{W}_{2}}>{{W}_{3}}\]
done
clear
D)
\[{{W}_{1}}>{{W}_{3}}>{{W}_{2}}\]
done
clear
View Answer play_arrow
question_answer 24) The gas is expanded in such a way so that its pressure and volume laws follow\[p{{V}^{2}}=\]constant. In this process, the gas will become
A)
hot
done
clear
B)
cold
done
clear
C)
nor hot neither cold
done
clear
D)
first hot after that cold
done
clear
View Answer play_arrow
question_answer 25) The time period of simple pendulum depends on
A)
length
done
clear
B)
mass
done
clear
C)
momentum
done
clear
D)
density
done
clear
View Answer play_arrow
question_answer 26) The stored uniform energy in a spring which is displaced by distance x is
A)
\[k/x\]
done
clear
B)
\[\frac{1}{2}\,k/{{x}^{2}}\]
done
clear
C)
\[k/{{x}^{2}}\]
done
clear
D)
\[2k/{{x}^{2}}\]
done
clear
View Answer play_arrow
question_answer 27) A particle of mass 0.10 kg is executing simple harmonic motion at the rate of 20 oscillation/\[{{s}^{2}}\] and its amplitude is 0.05 m. Its energy at equilibrium position will be
A)
2 J
done
clear
B)
4 J
done
clear
C)
1 J
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 28) When any rigid body is in rotational motion about any axis then what is the same for all particles?
A)
Angular velocity
done
clear
B)
Linear velocity
done
clear
C)
Radius
done
clear
D)
Linear acceleration
done
clear
View Answer play_arrow
question_answer 29) The coefficients of thermal conductivities of copper, murcury and glass respectively are\[{{K}_{c}},{{K}_{m}}\] and\[{{K}_{g}}\]such that\[{{K}_{c}}>\text{ }{{K}_{m}}>\text{ }{{K}_{g}}\]. If the same quantity of heat flows per second per unit area of each and corresponding temperature gradients are\[{{X}_{c}},{{X}_{m}}\]and\[{{X}_{g}},\]then
A)
\[{{X}_{c}}=\text{ }{{X}_{m}}=\text{ }{{X}_{g}}\]
done
clear
B)
\[{{X}_{c}}>{{X}_{m}}>\text{ }{{X}_{g}}\]
done
clear
C)
\[{{X}_{c}}<{{X}_{m}}<\text{ }{{X}_{g}}\]
done
clear
D)
\[{{X}_{m}}<{{X}_{c}}<\text{ }{{X}_{g}}\]
done
clear
View Answer play_arrow
question_answer 30) The emitted energy from any body depends on
A)
temperature
done
clear
B)
nature of matter
done
clear
C)
area
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 31) What is the absorptive power of ideal black body?
A)
0
done
clear
B)
1
done
clear
C)
\[\infty \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 32) The initial coordinates of any system are p and V. If a gas \[\left( \gamma =\frac{5}{3} \right)\]is compressed to \[\frac{1}{8}\] of its original volume, then the pressure of the gas will be
A)
p
done
clear
B)
16p
done
clear
C)
4p
done
clear
D)
32 p
done
clear
View Answer play_arrow
question_answer 33) Which of the following relationships between p and T for adiabatic process is true?
A)
\[{{p}^{1-\gamma }}+{{T}^{\gamma }}\] = constant
done
clear
B)
\[{{p}^{\gamma }}+{{T}^{1-\gamma }}\] = constant
done
clear
C)
\[p{{T}^{1-\gamma }}T\]= constant
done
clear
D)
\[{{p}^{1-\gamma }}T\]= constant
done
clear
View Answer play_arrow
question_answer 34) The energy related per degree of freedom of one molecule is
A)
\[\frac{3}{2}kT\]
done
clear
B)
\[\frac{1}{2}kT\]
done
clear
C)
\[\frac{3}{2}RT\]
done
clear
D)
\[\frac{1}{2}RT\]
done
clear
View Answer play_arrow
question_answer 35) Absolute temperature is that temperature at which
A)
molecular motion of all particles becomes zero
done
clear
B)
molecules move randomly
done
clear
C)
gas's atoms change to liquid
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 36) If the surface tension of liquid is\[5\times {{10}^{-2}}N/m\] and weight of liquid column is\[6.28\times {{10}^{-4}}N,\]then radius of capillary is
A)
\[2\times {{10}^{-3}}m\]
done
clear
B)
\[2.5\times {{10}^{-3}}m\]
done
clear
C)
\[2\times {{10}^{-4}}\]
done
clear
D)
\[4\times {{10}^{-3}}m\]
done
clear
View Answer play_arrow
question_answer 37) When the liquid does not wet the sides of a solid, then angle of contact is
A)
obtuse
done
clear
B)
acute
done
clear
C)
\[90{}^\circ \]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 38) A tuning fork is placed on the table. It produces maximum sound due to
A)
beat
done
clear
B)
resonance
done
clear
C)
interference
done
clear
D)
stationary waves
done
clear
View Answer play_arrow
question_answer 39) A body cools down from\[61{}^\circ C\]to\[59{}^\circ C\]in 4 min. If the temperature of atmosphere is\[30{}^\circ C,\]then the time taken to cool it from\[51{}^\circ C\]to\[49{}^\circ C\]will be
A)
4 min
done
clear
B)
2 min
done
clear
C)
6 min
done
clear
D)
8 min
done
clear
View Answer play_arrow
question_answer 40) Temperature is a measurement of degree of coldness or hotness of an object. The definition is based on
A)
Zeroth law of thermodynamics
done
clear
B)
First law of thermodynamics
done
clear
C)
Second law of thermodynamics
done
clear
D)
Newton's law of cooling
done
clear
View Answer play_arrow
question_answer 41) The correct relation between isothermal gradient and adiabatic gradient is
A)
adiabatic gradient\[=\gamma \times \] isothermal gradient
done
clear
B)
isothermal gradient\[=\gamma \times \] adiabatic gradient
done
clear
C)
adiabatic gradient\[={{\gamma }^{2}}\times \] isothermal gradient
done
clear
D)
isothermal gradient\[={{\gamma }^{2}}\times \]adiabatic gradient
done
clear
View Answer play_arrow
question_answer 42) If root mean square velocity for hydrogen gas is 318 m/s and density is\[8.99\times {{10}^{-5}}kg/{{m}^{3}}\]then the pressure of gas will be
A)
3 atm
done
clear
B)
1 atm
done
clear
C)
2 atm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 43) \[pV/kT\] represents
A)
number of molecules
done
clear
B)
number of moles
done
clear
C)
universal gas constant
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 44) The spherical shape of rain drop is due to
A)
surface tension
done
clear
B)
viscosity
done
clear
C)
elasticity
done
clear
D)
gravity
done
clear
View Answer play_arrow
question_answer 45) The excess of pressure inside a soap bubble than that of the outer pressure is
A)
\[\frac{4T}{r}\]
done
clear
B)
\[\frac{2T}{r}\]
done
clear
C)
\[\frac{8T}{r}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 46) There is an electric field E in\[x-\]direction. If the work done on moving a charge 0.2 C through a distance of 2 m along a line making an angle \[60{}^\circ \] with the\[x-\]axis is 4.0, what is the value of E?
A)
\[\sqrt{3}\]N/C
done
clear
B)
4N/C
done
clear
C)
5 N/C
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 47) An electron is accelerated from a potential difference of\[{{10}^{4}}V\]at distance 5 cm. The force on electron will be
A)
\[3.2\,\times \,{{10}^{-14}}N\]
done
clear
B)
\[4N\]
done
clear
C)
\[3.2\,\times \,{{10}^{-12}}N\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 48) A point charge\[+q\]is placed at the centre of a cube. The electric flux emerging from the cube
A)
\[\frac{q}{{{\varepsilon }_{0}}}\]
done
clear
B)
\[\frac{q}{2{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{q}{6{{\varepsilon }_{0}}}\]
done
clear
D)
\[\frac{q}{3{{\varepsilon }_{0}}}\]
done
clear
View Answer play_arrow
question_answer 49) To cool a liquid rapidly, cooling system should be used
A)
in middle
done
clear
B)
on head
done
clear
C)
any point
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 50) A stone is shot straight upward with a speed of 20 m/s from a tower 200 m high. The speed with which it strikes the ground is approximately
A)
60 m/s
done
clear
B)
65 m/s
done
clear
C)
70 m/s
done
clear
D)
75 m/s
done
clear
View Answer play_arrow
question_answer 51) When light ray goes from air to water, then its quality that remains unchanged is
A)
frequency
done
clear
B)
wavelength
done
clear
C)
speed
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 52) Sources are in phase when
A)
first phase is constant with the time
done
clear
B)
first phase changes with the time
done
clear
C)
first phase is constant with the displacement
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 53) Find the fundamental frequency of a closed pipe, if the length of pipe is 1 m. (speed of sound in air = 320 m/s)
A)
320 Hz
done
clear
B)
160 Hz
done
clear
C)
80 Hz
done
clear
D)
40 Hz
done
clear
View Answer play_arrow
question_answer 54) The musical interval between two tones of frequencies 400 Hz and 200 Hz is
A)
2
done
clear
B)
200
done
clear
C)
1
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 55)
Two pulses in a stretched string whose centres are initially 8 cm apart are moving towards each other as shown in the figure. The speed of each pulse is 2 cm/s.
A)
zero
done
clear
B)
purely kinetic
done
clear
C)
purely potential
done
clear
D)
partly kinetic and partly potential
done
clear
View Answer play_arrow
question_answer 56) Two charges of\[3\times {{10}^{-9}}\]and\[{{10}^{-9}}C\]are placed at a distance 5 cm. The force of attraction between them is
A)
\[1.08\times {{10}^{5}}N\]
done
clear
B)
\[1.08\times {{10}^{-6}}N\]
done
clear
C)
\[1.08\times {{10}^{-5}}N\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 57) If in diffraction by single slit, the width of slit is equal to wavelength of light, then what happened at the screen?
A)
Image of slit
done
clear
B)
Diffraction band
done
clear
C)
Equal illuminate
done
clear
D)
Unequal illuminate
done
clear
View Answer play_arrow
question_answer 58) Two positive point charges 12\[\mu \]C are 8\[\mu \]C are placed at a distance work done to bring closer 4 cm will be
A)
5.8 J
done
clear
B)
5.8 eV
done
clear
C)
13 J
done
clear
D)
13 eV
done
clear
View Answer play_arrow
question_answer 59) The object at distance of 20 cm is placed in front of convex lens of focal length 10 cm, where will be image formed?
A)
10 cm
done
clear
B)
20 cm
done
clear
C)
5 cm
done
clear
D)
25 cm
done
clear
View Answer play_arrow
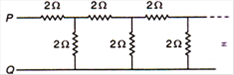
question_answer 60)
The equivalent resistance between points P and Q of an infinite network of resistance each of 2\[\Omega \] connected as shown in figure
A)
3.23\[\Omega \]
done
clear
B)
4.23\[\Omega \]
done
clear
C)
6.32\[\Omega \]
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 61) Two tuning forks of frequencies 256 and 258 Hz produce 5 beats/s with the third tuning fork. The frequency of third tuning fork will be
A)
120 Hz
done
clear
B)
115 Hz
done
clear
C)
105 Hz
done
clear
D)
95 Hz
done
clear
View Answer play_arrow
question_answer 62) The equation of wave is\[y=5\text{ }sin\text{ }n\text{ }(t+4)\]. The amplitude and time period will be
A)
5 cm, 2s
done
clear
B)
5 cm, \[\frac{1}{2}\]s
done
clear
C)
5 cm, 4s
done
clear
D)
5 cm, \[\frac{1}{4}\]s
done
clear
View Answer play_arrow
question_answer 63) If\[R=25\text{ }\Omega ,\text{ }Z=5\,\Omega \]then coefficient of power will be
A)
\[\frac{1}{2}\]
done
clear
B)
2
done
clear
C)
1
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 64) The formula of impedance for circuit R-L is
A)
\[\sqrt{{{R}^{2}}+\frac{1}{{{\omega }^{2}}{{L}^{2}}}}R\]
done
clear
B)
\[{{R}^{2}}+{{\omega }^{2}}{{L}^{2}}\]
done
clear
C)
\[\sqrt{{{R}^{2}}+{{\omega }^{2}}{{L}^{2}}}\]
done
clear
D)
\[\sqrt{{{\omega }^{2}}{{L}^{2}}+\frac{1}{{{R}^{2}}}}\,\]
done
clear
View Answer play_arrow
question_answer 65) Loss of power m R-L circuit is
A)
\[\frac{{{V}^{2}}}{({{R}^{2}}+{{\omega }^{2}}{{L}^{2}}}R\]
done
clear
B)
\[\frac{{{V}^{2}}}{\sqrt{{{R}^{2}}+{{\omega }^{2}}{{L}^{2}}}}\]
done
clear
C)
\[\frac{{{V}^{2}}}{R}\sqrt{{{R}^{2}}+{{\omega }^{2}}{{L}^{2}}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 66) The phase difference in R-C circuit will be
A)
\[\phi ={{\tan }^{-1}}\left( \frac{1}{\omega CR} \right)\]
done
clear
B)
\[\phi ={{\tan }^{-1}}\left( \frac{\omega }{RC} \right)\]
done
clear
C)
\[\phi ={{\tan }^{-1}}\left( \frac{RC}{\omega } \right)\]
done
clear
D)
\[\phi ={{\tan }^{-1}}\sqrt{\frac{\omega }{RC}}\]
done
clear
View Answer play_arrow
question_answer 67) The electric field gets induced by changing magnetic force lines passing through a conductor. This can be understood by which law?
A)
Faraday's law
done
clear
B)
Ampere's law
done
clear
C)
Lenz's law
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 68) In a transformer,\[{{e}_{p}}=110\text{ }V\]and\[{{e}_{s}}=440\text{ }V,\]then its round ratio will be
A)
4 : 1
done
clear
B)
1 : 4
done
clear
C)
1 : 3
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 69) The intensity of magnetic field at centre of circular coil is
A)
\[\frac{{{\mu }_{0}}I}{2R}\]
done
clear
B)
\[\frac{{{\mu }_{0}}NI{{R}^{2}}}{2{{({{R}^{2}}+{{x}^{2}})}^{3/2}}}\]
done
clear
C)
\[\frac{{{\mu }_{0}}I}{4R}\]
done
clear
D)
\[\frac{{{\mu }_{0}}I}{8R}\]
done
clear
View Answer play_arrow
question_answer 70) In a potentiometer experiment two cells of emf? s \[{{E}_{1}}\]and\[{{E}_{2}}\]are used in series and in conjunction and the balancing length is found to be 58 cm of the wire. If the polarity of\[{{E}_{2}}\]is reversed, then the balancing length becomes 29 cm. The ratio \[\frac{{{E}_{1}}}{{{E}_{2}}}\]of the emf of the two cells is
A)
1 : 1
done
clear
B)
2 : 1
done
clear
C)
3 : 1
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 71) Power dissipated in pure inductive circuit will be
A)
\[\frac{{{V}^{2}}}{({{R}^{2}}+{{\omega }^{2}}{{L}^{2}})}R\]
done
clear
B)
zero
done
clear
C)
\[\frac{{{V}^{2}}}{{{L}^{2}}}R\]
done
clear
D)
\[\frac{{{V}^{2}}}{{{Z}^{2}}}R\]
done
clear
View Answer play_arrow
question_answer 72) An AC circuit has voltage\[V=5\text{ }sin\text{ }10t,\]if\[R=1\,\Omega \]and\[L=0.1\text{ }H,\]then what is the phase difference between current and voltage?
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[0\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\infty \]
done
clear
View Answer play_arrow
question_answer 73) In R-L-C circuit,\[R=100\text{ }\Omega ,\text{ }L=1\text{ }H,\text{ }C=10\mu F\] and \[\omega \]= 314 rad /s, then find the value of impedance.
A)
100 \[\Omega \]
done
clear
B)
272.6 \[\Omega \]
done
clear
C)
217.2 \[\Omega \]
done
clear
D)
205.5\[\Omega \]
done
clear
View Answer play_arrow
question_answer 74) In circuit, when the current changes to 8A from 2A in 2 s, then the value of emf becomes 2 V. The inductance of the circuit is
A)
\[\frac{4}{3}H\]
done
clear
B)
\[\frac{2}{3}H\]
done
clear
C)
\[\frac{1}{3}H\]
done
clear
D)
\[\sqrt{\frac{2}{3}}\,H\]
done
clear
View Answer play_arrow
question_answer 75) Generator generates electric current. In actual, it is a source of
A)
inducted force
done
clear
B)
emf
done
clear
C)
electric force
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 76) Lenz's law is accordance on
A)
conservation of energy
done
clear
B)
conservation of charge
done
clear
C)
conservation of momentum
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 77) When the current flows from the conductor, then force above of magnetic field is
A)
in circular form around the wire
done
clear
B)
near the wire and parallel to wire
done
clear
C)
near the wire and perpendicular to wire
done
clear
D)
None of the above
done
clear
View Answer play_arrow
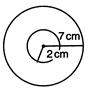
question_answer 78)
In the following figure, a coil of radius 2 cm is shown along with a coil of radius 7 cm present at its centre. Each coil has 100 round and big coil has 5 A current. What should be the current in small coil so that total magnetic field at centre is 2 mT?
A)
1.44 A
done
clear
B)
0.793 A
done
clear
C)
2.88 A
done
clear
D)
3.4 A
done
clear
View Answer play_arrow
question_answer 79) If amplification factor and mutual conductance are 20 and\[{{10}^{-3}}\]mho respectively, then plate resistance will be
A)
20 k\[\Omega \]
done
clear
B)
\[10\,k\Omega \]
done
clear
C)
\[20\times {{10}^{-3}}\Omega \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 80) On the basis which of the following a nucleus can be explained?
A)
By nuclear liquid drop model
done
clear
B)
By Thomson model
done
clear
C)
By Rutherford model
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 81) If the radius of nucleus is\[{{10}^{-15}}m,\]the uncertainty in momentum of nuclear particle will be
A)
\[\frac{6.62\,\times \,{{10}^{-19}}}{4\pi }\]
done
clear
B)
\[6.62\,\times \,{{10}^{-29}}\]
done
clear
C)
\[6.62\,\times \,{{10}^{-39}}\]
done
clear
D)
\[6.62\,\times \,{{10}^{-49}}\]
done
clear
View Answer play_arrow
question_answer 82) If de-Broglie wavelength and accelerated potential difference are 6000\[\overset{o}{\mathop{\text{A}}}\,\]and 9000 V respectively, then value of \[\frac{h}{e}\] will be
A)
\[1.9\times {{10}^{-10}}\]
done
clear
B)
\[18\,\times \,{{10}^{-19}}\]
done
clear
C)
\[8.2\,\times \,{{10}^{-20}}\]
done
clear
D)
\[18\,\times \,{{10}^{-20}}\]
done
clear
View Answer play_arrow
question_answer 83) Photoelectric effect will occur if
A)
\[v<{{v}_{0}}\]
done
clear
B)
\[v\ge {{v}_{0}}\]
done
clear
C)
\[v>2{{v}_{0}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 84) Increasing the principle quantum number, the energy gap between consequence energy state
A)
increases
done
clear
B)
decreases
done
clear
C)
remains unchange
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 85) First law of Kirchhoff is accordance on
A)
conservation of charge
done
clear
B)
conservation of energy
done
clear
C)
conservation of momentum
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 86) Three capacitors of equal capacitances 3 \[\mu \]F each are connected in a circuit. Then their maximum and minimum capacities will be
A)
\[9\mu F,1\mu F\]
done
clear
B)
\[8\mu F,2\mu F\]
done
clear
C)
\[9\mu F,0\mu F\]
done
clear
D)
\[3\mu F,2\mu F\]
done
clear
View Answer play_arrow
question_answer 87) For non-conductors, the forbidden energy gap is
A)
5 eV
done
clear
B)
1.1 eV
done
clear
C)
20 eV
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 88) Change of AC to DC is called
A)
rectification
done
clear
B)
polarization
done
clear
C)
amplification
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 89) Write the resolving powers of\[\alpha ,\beta \]and\[\gamma \]to ascending order.
A)
\[\alpha ,\beta ,\gamma \]
done
clear
B)
\[\gamma ,\beta ,\alpha \]
done
clear
C)
\[\beta ,\alpha ,\gamma \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 90) Diffraction of electron beam is proved by
A)
Davisson-Germer
done
clear
B)
Berg
done
clear
C)
Newton
done
clear
D)
Einsteen
done
clear
View Answer play_arrow
question_answer 91) On the basis of which photoelectric effect is explained?
A)
Relativity theory
done
clear
B)
The electromagnetic waves of light
done
clear
C)
Energy spectrum of atoms
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 92) Which of the following atoms or ions has minimum radius?
A)
H
done
clear
B)
\[{{H}^{+}}\]
done
clear
C)
\[L{{i}^{++}}\]
done
clear
D)
Deuteron
done
clear
View Answer play_arrow
question_answer 93) The value of resistance R will be
A)
10\[\Omega \]
done
clear
B)
15\[\Omega \]
done
clear
C)
20\[\Omega \]
done
clear
D)
25\[\Omega \]
done
clear
View Answer play_arrow
question_answer 94) What will be .the stored energy in capacitor of R-C
A)
\[\frac{CV}{2}\]
done
clear
B)
\[\frac{C{{V}^{2}}}{2}\]
done
clear
C)
\[\frac{{{q}^{2}}}{C}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 95) The valancy of carbon atom is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 96) What is valancy of impurity added for donar atoms?
A)
Pentavalent
done
clear
B)
Trivalent
done
clear
C)
Tertravalent
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 97) In LC-R circuit,\[R=300\Omega ,\text{ }L=0.9\text{ }H,\text{ }C=\]\[2\mu F\]and \[\omega =1000\]rad/s, the impedance of circuit is
A)
1300\[\Omega \]
done
clear
B)
900\[\Omega \]
done
clear
C)
500\[\Omega \]
done
clear
D)
400\[\Omega \]
done
clear
View Answer play_arrow
question_answer 98) What should be the capacity of capacitor of R-C circuit, in which the value of resistance is 10\[\Omega \] to become the value of time constant 10?
A)
\[10\mu F\]
done
clear
B)
\[100\mu F\]
done
clear
C)
\[1000\mu F\]
done
clear
D)
\[10,000\mu F\]
done
clear
View Answer play_arrow
question_answer 99) The energy needed to remove the one electron from neutral helium atom is 24.6 eV. Then the energy needed to remove both the electrons from neutral helium atom is
A)
79.0 eV
done
clear
B)
51.8 eV
done
clear
C)
49.2 eV
done
clear
D)
38.2 eV
done
clear
View Answer play_arrow
question_answer 100) Two same light cathode gets the light of frequencies\[{{f}_{1}}\]and\[{{f}_{2}}\]. If the velocities of emitted photo electrons are \[{{v}_{1}}\] and \[{{v}_{2}}\] then
A)
\[{{v}^{2}}_{1}-v\frac{2}{2}=\frac{2h}{m}({{f}_{1}}-{{f}_{2}})\]
done
clear
B)
\[{{v}_{1}}+{{v}_{2}}={{\left[ \frac{2h}{m}({{f}_{1}}+{{f}_{2}}) \right]}^{2}}\]
done
clear
C)
\[{{v}^{2}}_{1}+{{v}^{2}}_{2}=\frac{2h}{m}({{f}_{1}}+{{f}_{2}})\]
done
clear
D)
\[{{v}_{1}}-{{v}_{2}}={{\left[ \frac{2h}{m}({{f}_{1}}-{{f}_{2}} \right]}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 101) For the salt of weak acid and weak base, the value of\[{{K}_{b}}\]will be
A)
\[{{K}_{b}}=\frac{{{K}_{w}}}{{{K}_{a}}}\]
done
clear
B)
\[{{K}_{h}}=\frac{{{K}_{w}}}{{{K}_{b}}}\]
done
clear
C)
\[{{K}_{h}}=\frac{{{K}_{w}}}{{{K}_{a}}.{{K}_{b}}}\]
done
clear
D)
Hydrolysis does not take place
done
clear
View Answer play_arrow
question_answer 102) Formation of cyanohydrin from\[C{{H}_{3}}COC{{H}_{3}}\]is called
A)
electrophilic substitution
done
clear
B)
nucleophilic substitution
done
clear
C)
nucleophilic addition
done
clear
D)
electrophilic substitution
done
clear
View Answer play_arrow
question_answer 103) Bond energy of\[C-H\]bond in ethane, ethane and ethyne is
A)
equal in three
done
clear
B)
more in ethane
done
clear
C)
more in ethene
done
clear
D)
more in ethyne
done
clear
View Answer play_arrow
question_answer 104) \[C{{H}_{3}}-CH=C{{H}_{2}}+HBr\xrightarrow[{}]{{}}A;\] compound A is
A)
\[\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,-CH=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}Br\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
done
clear
D)
\[\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,\]
done
clear
View Answer play_arrow
question_answer 105) Which of the following is represented by prefix word 'alkali' used for alkali metals?
A)
Ash of plants
done
clear
B)
Lusture of silver
done
clear
C)
Metallic nature
done
clear
D)
Reactive metal
done
clear
View Answer play_arrow
question_answer 106) The product is obtained on reaction of urea with ammonia
A)
barbituric acid
done
clear
B)
semicarbazide
done
clear
C)
methylol urea
done
clear
D)
acetamide
done
clear
View Answer play_arrow
question_answer 107) Structure of ammonia is
A)
pyramidal
done
clear
B)
tetrahederal
done
clear
C)
trigonal
done
clear
D)
trigonal bipyramidal
done
clear
View Answer play_arrow
question_answer 108) The decreasing order of reactivity of alkyl halide is
A)
\[RI>RCl>RBr\]
done
clear
B)
\[RCl>RI>RBr\]
done
clear
C)
\[RCl>RBr>RI\]
done
clear
D)
\[RI>RBr>RCl\]
done
clear
View Answer play_arrow
question_answer 109) If the pH value of\[HCl\]solution is 4 then its molar concentration will
A)
4 M
done
clear
B)
0.0001 M
done
clear
C)
0.4 M
done
clear
D)
10 M
done
clear
View Answer play_arrow
question_answer 110) The product is formed on the condensation of phenol with phthalic anhydride
A)
methyl orange
done
clear
B)
phenol red
done
clear
C)
salicylic acid
done
clear
D)
phenolphthalein
done
clear
View Answer play_arrow
question_answer 111) 23 g sodium metal reacts from methyl alcohol to give
A)
1 mol\[{{H}_{2}}\]
done
clear
B)
2 mol\[{{H}_{2}}\]
done
clear
C)
\[\frac{1}{2}\] mol\[{{H}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 112) Nitration of benzene is
A)
electrophilic addition
done
clear
B)
electrophilic substitution
done
clear
C)
nucleophilic addition
done
clear
D)
nucleophilic substitution
done
clear
View Answer play_arrow
question_answer 113) Which of the following has maximum magnetic moment?
A)
\[3{{d}^{5}}\]
done
clear
B)
\[3{{d}^{2}}\]
done
clear
C)
\[3{{d}^{9}}\]
done
clear
D)
\[3{{d}^{7}}\]
done
clear
View Answer play_arrow
question_answer 114) The correct order of atomic and ionic size of iodine is
A)
\[I>{{I}^{-}}>{{I}^{+}}\]
done
clear
B)
\[{{I}^{-}}>I>{{I}^{+}}\]
done
clear
C)
\[I>{{I}^{+}}>{{I}^{-}}\]
done
clear
D)
\[{{I}^{+}}>I>{{I}^{-}}\]
done
clear
View Answer play_arrow
question_answer 115) Number of\[\sigma \]and\[\pi \]bonds in l-butene-3-yne
A)
\[5\sigma ,5\pi \]
done
clear
B)
\[7\sigma ,3\pi \]
done
clear
C)
\[8\sigma ,6\pi \]
done
clear
D)
\[6\sigma ,4\pi \]
done
clear
View Answer play_arrow
question_answer 116) Which of the following salt does not give brown gas on heating?
A)
\[LiN{{O}_{3}}\]
done
clear
B)
\[KN{{O}_{3}}\]
done
clear
C)
\[Pb{{(N{{O}_{3}})}_{2}}\]
done
clear
D)
\[AgN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 117) 2-chloropropane reacts with sodium in presence of ether to form
A)
n-hexane
done
clear
B)
n-hexene
done
clear
C)
\[C{{H}_{3}}-CH=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,=C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 118) \[{{C}_{6}}{{H}_{6}}+C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-Cl\xrightarrow[{}]{{}}A;\]Product A is
A)
benzophenone
done
clear
B)
benzoyl chloride
done
clear
C)
acetophenone
done
clear
D)
toluene
done
clear
View Answer play_arrow
question_answer 119) Which group is/are present in picric acid?
A)
\[-OH\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[-OH\] and\[-N{{O}_{2}}\]
done
clear
D)
\[-COOH\]and\[-N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 120) Number of\[C-C\]and\[C-H\]bonds in 4-methyl-2-pentyne will be
A)
4, 10
done
clear
B)
5, 10
done
clear
C)
4, 9
done
clear
D)
5, 9
done
clear
View Answer play_arrow
question_answer 121) The oxidation state of iron is zero in which of the following compound?
A)
\[FeS{{O}_{4}}\]
done
clear
B)
\[Fe{{(CO)}_{4}}\]
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
done
clear
D)
\[FeC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 122) Which of the following overlapping does not give\[\pi -\]bond in a molecule AB? (z-axis is the axis of molecule)
A)
\[{{p}_{x}}-{{p}_{x}}\]
done
clear
B)
\[{{p}_{y}}-{{p}_{xy}}\]
done
clear
C)
\[{{p}_{y}}-s\]
done
clear
D)
\[{{p}_{y}}-{{p}_{y}}\]
done
clear
View Answer play_arrow
question_answer 123) Which of the following equilibrium does not affect from change in pressure?
A)
\[2N{{O}_{2}}(g){{N}_{2}}{{O}_{4}}(g)\]
done
clear
B)
\[2HI(g){{H}_{2}}(g)+{{I}_{2}}(g)\]
done
clear
C)
\[2{{O}_{3}}(g)3{{O}_{2}}(g)\]
done
clear
D)
\[PC{{l}_{5}}(g)PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 124) The concentration of a acidic solution is 0.1 M and degree of dissociation is 1%. The dissociation constant of acid will be
A)
\[1.01\times {{10}^{-5}}\]
done
clear
B)
\[1.01\times {{10}^{-3}}\]
done
clear
C)
\[1.01\times {{10}^{3}}\]
done
clear
D)
\[1.01\times {{10}^{5}}\]
done
clear
View Answer play_arrow
question_answer 125) Number of elements in s-block are
A)
more from elements of p-block
done
clear
B)
less from elements of d-block
done
clear
C)
more from elements of d-block
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 126) On doubling the concentration of A and B in reaction\[A+BAB,\]the equilibrium constant will be
A)
unchanged
done
clear
B)
halved
done
clear
C)
doubled
done
clear
D)
four times
done
clear
View Answer play_arrow
question_answer 127) The compound is not used in redox titration
A)
\[KMn{{O}_{4}}\]
done
clear
B)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
C)
\[{{(COOH)}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 128) Which of the following does not used as primary standard in redox titration?
A)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
B)
\[KMn{{O}_{4}}\]
done
clear
C)
\[{{(COOH)}_{2}}\]
done
clear
D)
Mohr's salt
done
clear
View Answer play_arrow
question_answer 129) The solubility product of a metal sulphide MS is\[7\times {{10}^{-24}}mo{{l}^{2}}{{L}^{-2}}\]. The solubility of MS will be
A)
\[7\times {{10}^{-24}}mo{{l}^{2}}{{L}^{2}}\]
done
clear
B)
\[2.65\times {{10}^{-12}}mol\,{{L}^{-1}}\]
done
clear
C)
\[1.32\times {{10}^{-12}}mol\,{{L}^{-1}}\]
done
clear
D)
\[14\times {{10}^{-24}}mo{{l}^{3}}{{L}^{2}}\]
done
clear
View Answer play_arrow
question_answer 130) Which ion has the maximum hydration energy?
A)
\[M{{g}^{2+}}\]
done
clear
B)
\[B{{a}^{2+}}\]
done
clear
C)
\[C{{a}^{2+}}\]
done
clear
D)
\[S{{r}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 131) What will form on reduction of\[{{P}_{2}}{{O}_{5}}\]from acetic acid?
A)
Acetic anhydride
done
clear
B)
Acetyl chloride
done
clear
C)
Acetic ester
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 132) Possible number of isomers of alkane\[{{C}_{5}}{{H}_{12}}\]will be
A)
4
done
clear
B)
3
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 133) Molecular formula of chrornyl chloride is
A)
\[Cr{{O}_{2}}C{{l}_{2}}\]
done
clear
B)
\[Cr{{O}_{2}}Cl\]
done
clear
C)
\[CrOC{{l}_{2}}\]
done
clear
D)
\[{{(CrOCl)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 134) Phenol reacts with dil.\[HN{{O}_{3}}\]to give
A)
o-nitrophenol
done
clear
B)
p-nitrophenol
done
clear
C)
o- and p-nitrophenol
done
clear
D)
picric acid
done
clear
View Answer play_arrow
question_answer 135) Which of the following compound is obtained from ozonolysis of benzene?
A)
Benzene triozonide
done
clear
B)
Glyoxal
done
clear
C)
Ethane dial
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 136) Oxidation state of iron and sulphur in copper pyrite are respectively
A)
+ 2 and -2
done
clear
B)
+ 2 and - 4
done
clear
C)
+ 3 and -4
done
clear
D)
+ 3 and - 6
done
clear
View Answer play_arrow
question_answer 137) What will obtain on distillation of acetic acid with\[PC{{l}_{3}}\]?
A)
Acetic anhydride
done
clear
B)
Chloroacetic acid
done
clear
C)
Acetyl chloride
done
clear
D)
Ethyl acetate
done
clear
View Answer play_arrow
question_answer 138) Strongest acid is
A)
\[C{{l}_{2}}CH.CHOOH\]
done
clear
B)
\[C{{H}_{3}}COOH\]
done
clear
C)
\[CC{{l}_{3}}COOH\]
done
clear
D)
\[ClC{{H}_{2}}.COOH\]
done
clear
View Answer play_arrow
question_answer 139) The gas is released in fermentation reaction
A)
\[{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}\]
done
clear
D)
\[S\]
done
clear
View Answer play_arrow
question_answer 140) Which of the following molecule of compound will be linear structure?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 141) Which of the following ion is colourless?
A)
\[C{{u}^{+}}\]
done
clear
B)
\[C{{u}^{2+}}\]
done
clear
C)
\[C{{u}^{3+}}\]
done
clear
D)
\[F{{e}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 142) The colour of iodine solution disappears on reaction with which of the following compound?
A)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[CC{{l}_{4}}\]
done
clear
D)
\[CHC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 143) \[PC{{l}_{5}}\]is used in the
A)
synthesis of \[C{{H}_{3}}COCl\]
done
clear
B)
synthesis of \[{{C}_{6}}{{H}_{5}}OH\]
done
clear
C)
synthesis of \[{{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}\]
done
clear
D)
synthesis of \[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 144) It is cleared by the a-particle scattering experiment of Rutherford. That
A)
\[\alpha -\]particle is heavier than electron
done
clear
B)
\[\alpha -\]particle is positively charged
done
clear
C)
mostly part of atom is hollow
done
clear
D)
\[\alpha -\]particle moves with high velocity
done
clear
View Answer play_arrow
question_answer 145) Which of the following compound has maximum covalent character?
A)
\[NaF\]
done
clear
B)
\[CaC{{l}_{2}}\]
done
clear
C)
\[BeB{{r}_{2}}\]
done
clear
D)
\[KI\]
done
clear
View Answer play_arrow
question_answer 146) The suitable indicator in the titration of strong acid and weak base is
A)
methyl orange
done
clear
B)
bromothymol blue
done
clear
C)
phenol red
done
clear
D)
phenolphthalein
done
clear
View Answer play_arrow
question_answer 147) The product is formed on the reaction of ethylene bromide with Zn
A)
alkane
done
clear
B)
alkene
done
clear
C)
alkyne
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 148) Which of the following is formed on the hydrolysis of product, formed by the reaction of\[C{{H}_{3}}CHO\]and\[C{{H}_{3}}MgBr\]?
A)
Primary alcohol
done
clear
B)
Secondary alcohol
done
clear
C)
Tertiary alcohol
done
clear
D)
Ketone
done
clear
View Answer play_arrow
question_answer 149) Bond angle is\[s{{p}^{2}}\]hybridization is
A)
\[180{}^\circ \]
done
clear
B)
\[120{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[109{}^\circ 45'\]
done
clear
View Answer play_arrow
question_answer 150) Scientist who discovered the positron?
A)
Pauling
done
clear
B)
Anderson
done
clear
C)
Yukava
done
clear
D)
Sanger
done
clear
View Answer play_arrow
question_answer 151) Number of electrons are required for the complete reduction of one molecule of \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 152) Which of the following pair has same oxidation number of sulphur and chromium?
A)
\[SO_{3}^{2-},CrO_{4}^{2-}\]
done
clear
B)
\[S{{O}_{3}},CrO_{4}^{2-}\]
done
clear
C)
\[S{{O}_{2}},CrO_{4}^{2-}\]
done
clear
D)
\[S{{O}_{2}},C{{r}_{2}}O_{7}^{2-}\]
done
clear
View Answer play_arrow
question_answer 153) Which of the following gives haloform reaction?
A)
Acetaldehyde
done
clear
B)
Propionaldehyde
done
clear
C)
iso-butyraldehyde
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 154) The percentage of nitrogen will equal in oximes of which of the following compound pairs?
A)
Acetone and acetaldehyde
done
clear
B)
Acetone and propionaldehyde
done
clear
C)
Aqetone and acraldehyde
done
clear
D)
Acetaldehyde and acraldehyde
done
clear
View Answer play_arrow
question_answer 155) The highest atomic number radioactive element in p-block elements is
A)
Pb
done
clear
B)
Te
done
clear
C)
Rn
done
clear
D)
Po
done
clear
View Answer play_arrow
question_answer 156) Which of the following reagent evolves nitrogen on reaction with ethyl amine?
A)
Nitrosyl chloride
done
clear
B)
Acetyl chloride
done
clear
C)
Carbon disulphide
done
clear
D)
Benzoyl chloride
done
clear
View Answer play_arrow
question_answer 157) The density of ice is less than water because
A)
ice floats on water
done
clear
B)
the structure of ice is three dimensional with porous
done
clear
C)
H-bond does not present in ice
done
clear
D)
water is a polar solvent
done
clear
View Answer play_arrow
question_answer 158) The value of energy will increase in sub energy level because
A)
on increasing the value of principal quantum number
done
clear
B)
on increasing the value of azimuthal quantum number
done
clear
C)
on increasing the value of both principal quantum number and azimuthal quantum number
done
clear
D)
on increasing the value of spin quantum Number
done
clear
View Answer play_arrow
question_answer 159) The density of Na is higher than K because
A)
ionization potential of Na is greater than K
done
clear
B)
size of Na is smaller than K
done
clear
C)
atomic weight of K is greater than Na
done
clear
D)
only eight electrons are present in third shell of K
done
clear
View Answer play_arrow
question_answer 160) Same magnetic moment ions are
A)
\[C{{u}^{+}},F{{e}^{3+}}\]
done
clear
B)
\[M{{n}^{2+}},F{{e}^{3+}}\]
done
clear
C)
\[C{{u}^{2+}},F{{e}^{2+}}\]
done
clear
D)
\[M{{n}^{2+}},T{{i}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 161) The set of oxidation of nitrogen in ammonium nitrate is
A)
\[-3,+3\]
done
clear
B)
\[-1,+1\]
done
clear
C)
\[+1,-1\]
done
clear
D)
\[-3,+5\]
done
clear
View Answer play_arrow
question_answer 162) At\[300{}^\circ C,\]the vapour density of\[PC{{l}_{5}}\]is 60. At this temperature, its dissociation percentage will be
A)
73
done
clear
B)
77
done
clear
C)
83
done
clear
D)
87
done
clear
View Answer play_arrow
question_answer 163) The degree of dissociation of A is\[\alpha \]in equilibrium reaction\[A\xrightarrow[{}]{{}}2B+C\]. If one mole of A is taken initially then total .number of moles will in equilibrium.
A)
\[1-\alpha \]
done
clear
B)
\[1+\alpha \]
done
clear
C)
\[1-2\alpha \]
done
clear
D)
\[1+2\alpha \]
done
clear
View Answer play_arrow
question_answer 164) Which of the following compounds will form mesitylene on distillation with cone.\[{{H}_{2}}S{{O}_{4}}\]?
A)
Acetaldehyde
done
clear
B)
Acetone
done
clear
C)
Acetophenone
done
clear
D)
Acetyl chlorine
done
clear
View Answer play_arrow
question_answer 165) Generally, alkenes and alkynes show the following type of reaction
A)
nucleophilic addition
done
clear
B)
electrophilic addition
done
clear
C)
free radical substitution
done
clear
D)
electrophilic substitution
done
clear
View Answer play_arrow
question_answer 166) Which of the following is not a polymer?
A)
Teflon
done
clear
B)
Nylon
done
clear
C)
Orion
done
clear
D)
Phorone
done
clear
View Answer play_arrow
question_answer 167) The formula of wavelength of spectral lines for hydrogen atom is
A)
\[\frac{1}{\lambda }=R\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
done
clear
B)
\[\frac{1}{\lambda }=R\left( \frac{1}{n_{2}^{2}}-\frac{1}{n_{1}^{2}} \right)\]
done
clear
C)
\[\frac{1}{\lambda }=R{{\left( \frac{1}{{{n}_{1}}}-\frac{1}{{{n}_{2}}} \right)}^{2}}\]
done
clear
D)
\[\frac{1}{\lambda }=R{{\left( \frac{1}{{{n}_{2}}}-\frac{1}{{{n}_{1}}} \right)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 168) Power alcohol is
A)
absolute alcohol\[+C{{H}_{3}}OH\]
done
clear
B)
absolute alcohol\[+{{C}_{6}}{{H}_{5}}OH\]
done
clear
C)
absolute alcohol + petrol 4- benzene
done
clear
D)
absolute alcohol \[+C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 169) \[C{{H}_{3}}C{{H}_{2}}OH\]and\[C{{H}_{3}}-O-C{{H}_{3}}\]are
A)
positive isomers
done
clear
B)
functional isomers
done
clear
C)
chain isomers
done
clear
D)
geometrical isomers
done
clear
View Answer play_arrow
question_answer 170) Alkaline\[KMn{{O}_{4}}\]solution is called
A)
Tollen's reagent
done
clear
B)
Baeyer's reagent
done
clear
C)
Benedict's solution
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 171) The bond angle and bond length in benzene are respectively
A)
\[120{}^\circ \]and 1.34\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[120{}^\circ \]and 1.39\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[180{}^\circ \]and 1.33\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[120{}^\circ \]and 1.54\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 172) \[{{(C{{H}_{3}}CO)}_{2}}O+HCl\xrightarrow[{}]{{}}X+C{{H}_{3}}COH;\] compound X is
A)
\[C{{H}_{3}}Cl\]
done
clear
B)
\[C{{H}_{3}}COCl\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 173) The main product of the reaction of \[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]with nitrous acid is
A)
\[C{{H}_{3}}CN\]
done
clear
B)
\[C{{H}_{3}}ONO\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}-N=O\]
done
clear
View Answer play_arrow
question_answer 174) \[RX+2Na+BX\xrightarrow[{}]{{}}R-R+2NaX\] This reaction is known as
A)
Wurtz reaction
done
clear
B)
Williamson's synthesis
done
clear
C)
Kolbe's electrolysis
done
clear
D)
Sabatier-Sendern's reaction
done
clear
View Answer play_arrow
question_answer 175) In froth floatation method of ore concentration, ore particles rise to the surface because
A)
they are light
done
clear
B)
these are insoluble
done
clear
C)
their surface do not wet with water easily
done
clear
D)
these contain electric charge
done
clear
View Answer play_arrow
question_answer 176) The number of k electrons present in aromatic ring are
A)
\[(4+2)n\]
done
clear
B)
\[(4+2n)\]
done
clear
C)
\[(4n+2)\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 177) \[C{{H}_{3}}C{{H}_{2}}I+NaOR\to C{{H}_{3}}C{{H}_{2}}R+NaI\] This reaction is
A)
Wurtz reaction
done
clear
B)
Williamson's synthesis
done
clear
C)
Wittig reaction
done
clear
D)
Curtius reaction
done
clear
View Answer play_arrow
question_answer 178) Absolute alcohol can obtained from rectified spirit by the process of
A)
Steam distillation
done
clear
B)
Fractional distillation
done
clear
C)
Azeotropic distillation
done
clear
D)
Hydrolysis
done
clear
View Answer play_arrow
question_answer 179) The product of the reaction of benzene with\[CO+HCl\]in the presence of anhydrous\[AlC{{l}_{3}}\]is
A)
benzoyl chloride
done
clear
B)
benzal chloride
done
clear
C)
chlorobenzene
done
clear
D)
benzaldehyde
done
clear
View Answer play_arrow
question_answer 180) The suitable indicator for the titration of \[N{{H}_{4}}OH\]and\[HCl\]is
A)
phenolphthalein
done
clear
B)
methyl orange
done
clear
C)
bromothymol blue
done
clear
D)
litmus paper
done
clear
View Answer play_arrow
question_answer 181) Which of the following is not Lewis acid?
A)
\[FeC{{l}_{3}}\]
done
clear
B)
\[BC{{l}_{3}}\]
done
clear
C)
\[AlC{{l}_{3}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 182) Which of the following metal can displace Cu from copper sulphate solution?
A)
Fe
done
clear
B)
Ag
done
clear
C)
Au
done
clear
D)
Pt
done
clear
View Answer play_arrow
question_answer 183) Which enzyme is used for the conversion of starch into maltose?
A)
Zymase
done
clear
B)
Invertase
done
clear
C)
Maltase
done
clear
D)
Diastase
done
clear
View Answer play_arrow
question_answer 184) Which of the following is used in the extraction of copper?
A)
\[C{{u}_{2}}S\]
done
clear
B)
Pyrite
done
clear
C)
Silver argentocyanide
done
clear
D)
\[CuFe{{S}_{2}}\]
done
clear
View Answer play_arrow
question_answer 185) The product of the reaction of ethanol with ethyl magnesium iodide is
A)
\[C{{H}_{3}}-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}-C{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}-O-C{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 186) Which of the following element has the maximum tendency of loose the electron?
A)
Be
done
clear
B)
B
done
clear
C)
Mg
done
clear
D)
Rb
done
clear
View Answer play_arrow
question_answer 187) Which of the following is formed on the hydrolysis of the product, formed by the reaction of acetone with methyl magnesium iodide?
A)
Primary alcohol
done
clear
B)
Secondary alcohol
done
clear
C)
Tertiary alcohol
done
clear
D)
Carboxylic acid
done
clear
View Answer play_arrow
question_answer 188) Which complex forms on adding sodium cyanide solution in\[A{{g}_{2}}S,\]in the cyanide method of silver extraction?
A)
\[N{{a}_{2}}[Ag{{(CN)}_{2}}]\]
done
clear
B)
\[Na[Ag{{(CN)}_{2}}]\]
done
clear
C)
\[NaAgCN\]
done
clear
D)
\[Na[Ag{{(CN)}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 189) Benzene was discovered by
A)
Faraday
done
clear
B)
Prisetly
done
clear
C)
Kekule
done
clear
D)
Hofmann
done
clear
View Answer play_arrow
question_answer 190) Which substance is used in the preparation of absolute alcohol from rectified spirit?
A)
\[Ca{{(OH)}_{2}}\]
done
clear
B)
\[CaO\]
done
clear
C)
Anhydrous \[CaC{{l}_{2}}\]
done
clear
D)
Wire of Na
done
clear
View Answer play_arrow
question_answer 191) Benzenediazonium chloride reacts with phenol to give
A)
\[{{C}_{6}}{{H}_{5}}-NH-{{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}-NH=N-{{C}_{6}}{{H}_{5}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} \downarrow \\ O \end{smallmatrix}}{\mathop{N}}\,=N-{{C}_{6}}{{H}_{5}}\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 192) The first ionization potentials of nitrogen and oxygen are respectively
A)
14.6 eV, 13.6 eV
done
clear
B)
13.6 eV, 14.6 eV
done
clear
C)
13.6 eV, 13.6 eV
done
clear
D)
14.6 eV, 14.6 eV
done
clear
View Answer play_arrow
question_answer 193) Which of the following compound forms 3-hydroxy butanal on reaction with dil. alkali?
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[C{{H}_{3}}CHO+C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 194) The product of the reaction of ethylidene chloride with zinc dust is
A)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
B)
\[CH\equiv CH\]
done
clear
C)
\[C{{H}_{3}}-CH=CH-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 195) Which of the following pair shows the paramagnetic character?
A)
\[FeS{{O}_{4}}\]and\[NiC{{l}_{2}}\]
done
clear
B)
\[ZnC{{l}_{2}}\]and\[FeS{{O}_{4}}\]
done
clear
C)
\[MgC{{l}_{2}}\]and\[NiC{{l}_{4}}\]
done
clear
D)
\[KCl\]and\[ZnC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 196) The quantum number which shows the orientation of electron cloud in the presence of external magnetic field?
A)
Principal quantum number
done
clear
B)
Azimuthal quantum number
done
clear
C)
Magnetic quantum number
done
clear
D)
Spin quantum number
done
clear
View Answer play_arrow
question_answer 197) Oxidation number of\[Mn\]in\[MnO_{4}^{-}\]is
A)
+5
done
clear
B)
+ 6
done
clear
C)
+7
done
clear
D)
+ 4
done
clear
View Answer play_arrow
question_answer 198) Which of the following salt gives anionic hydrolysis?
A)
\[NaCl\]
done
clear
B)
\[NaCN\]
done
clear
C)
\[N{{H}_{4}}Cl\]
done
clear
D)
\[CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 199) Which of the following compounds will form chloropicrin on reaction with cone.\[HN{{O}_{3}}\]?
A)
\[CC{{l}_{4}}\]
done
clear
B)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 200) \[{{C}_{6}}{{H}_{5}}N{{O}_{2}}\xrightarrow[{}]{Zn+NaOH}\] Product The product of reaction is
A)
\[{{C}_{6}}{{H}_{5}}-NH-NH-{{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}-N=N-{{C}_{6}}{{H}_{5}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}-\underset{\downarrow }{\mathop{N}}\,={{C}_{6}}{{H}_{5}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 201) If the base of an equilateral triangle is\[x+y=2\]and vertex,\[(2,-1)\]is, then length of each side is equal to
A)
\[\sqrt{\frac{2}{3}}\]
done
clear
B)
\[\sqrt{\frac{3}{2}}\]
done
clear
C)
\[\frac{3}{\sqrt{2}}\]
done
clear
D)
\[\frac{2}{3}\]
done
clear
View Answer play_arrow
question_answer 202) Minimum number of zeros in an upper triangular matrix is
A)
\[\frac{n(n+1)}{2}\]
done
clear
B)
\[\frac{n(n-1)}{2}\]
done
clear
C)
\[\frac{2n(n-1)}{2}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 203) \[\left| \begin{matrix} {{1}^{2}} & {{2}^{2}} & {{3}^{2}} \\ {{2}^{2}} & {{3}^{2}} & {{4}^{2}} \\ {{3}^{2}} & {{4}^{2}} & {{5}^{2}} \\ \end{matrix} \right|\]is equal to
A)
8
done
clear
B)
\[-8\]
done
clear
C)
400
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 204) For any complex number z, minimum value of\[|z|+|z-1|\]is equal to
A)
1
done
clear
B)
0
done
clear
C)
1/2
done
clear
D)
3/2
done
clear
View Answer play_arrow
question_answer 205) \[\left| \begin{matrix} 1/a & {{a}^{2}} & bc \\ 1/b & {{b}^{2}} & ca \\ 1/c & {{c}^{2}} & ab \\ \end{matrix} \right|\]is equal to
A)
\[abc\]
done
clear
B)
0
done
clear
C)
1
done
clear
D)
\[a+b+c\]
done
clear
View Answer play_arrow
question_answer 206) lf\[^{n}{{C}_{3}}{{+}^{n}}{{C}_{4}}{{>}^{n+1}}{{C}_{3}},\]then
A)
\[n>7\]
done
clear
B)
\[n<6\]
done
clear
C)
\[n>6\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 207) If \[{{x}^{2}}+{{y}^{2}}=t-\frac{1}{t},{{x}^{4}}+{{y}^{4}}={{t}^{2}}+\frac{1}{{{t}^{2}}},\]then\[\frac{dy}{dx}\]is equal to
A)
\[\frac{1}{x{{y}^{3}}}\]
done
clear
B)
\[\frac{1}{{{x}^{3}}y}\]
done
clear
C)
\[-\frac{1}{x{{y}^{3}}}\]
done
clear
D)
\[-\frac{1}{{{x}^{3}}y}\]
done
clear
View Answer play_arrow
question_answer 208) \[\hat{i}\times (\hat{j}\times \hat{k})+\hat{j}\times (\hat{k}\times \hat{i})+\hat{k}(\hat{i}\times \hat{j})\]is equal to
A)
1
done
clear
B)
0
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 209) The area of a rectangle of given perimeter is minimum, if the ratio of its length to width is
A)
1:1
done
clear
B)
2:1
done
clear
C)
4:3
done
clear
D)
3:2
done
clear
View Answer play_arrow
question_answer 210) If AB = A, BA = B for any two square matrices A and B, then\[{{A}^{2}}\]is equal to
A)
A
done
clear
B)
B
done
clear
C)
AB
done
clear
D)
\[{{B}^{2}}\]
done
clear
View Answer play_arrow
question_answer 211) If\[z=x+iy,\]then \[z\overline{z}+2(z+\overrightarrow{z})+c=0\]will define a
A)
circle
done
clear
B)
straight line
done
clear
C)
parabola
done
clear
D)
point
done
clear
View Answer play_arrow
question_answer 212) If\[(3,\lambda )\]and (5,6) are the conjugate points of \[{{x}^{2}}+{{y}^{2}}=3,\]then \[\lambda \]is equal to
A)
2
done
clear
B)
\[-2\]
done
clear
C)
1
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 213) The argument of\[{{\left( \frac{1+\cos \theta +i\sin \theta }{1+\cos \theta -i\sin \theta } \right)}^{n}}\]is
A)
\[n\theta \]
done
clear
B)
\[\frac{n\theta }{2}\]
done
clear
C)
\[-\frac{n\theta }{2}\]
done
clear
D)
\[-n\theta \]
done
clear
View Answer play_arrow
question_answer 214) The figure made by the lines\[ax\pm by\pm c=0\]is
A)
rectangle
done
clear
B)
square
done
clear
C)
rhombus
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 215) If focal chord of a parabola\[{{y}^{2}}=4ax\]intercepted at b and k, then k is equal to
A)
\[\frac{ab}{b-a}\]
done
clear
B)
\[\frac{b}{b-a}\]
done
clear
C)
\[\frac{a}{b-a}\]
done
clear
D)
\[\frac{ba}{a-b}\]
done
clear
View Answer play_arrow
question_answer 216) If\[\overrightarrow{a}+\overrightarrow{b}+\overrightarrow{c}=0\]and\[|\overrightarrow{a}|=5,|\overrightarrow{b}|=3,|\overrightarrow{c}|=7,\]then angle between a and b is
A)
\[\frac{\pi }{6}\]
done
clear
B)
\[\frac{\pi }{4}\]
done
clear
C)
\[\frac{\pi }{3}\]
done
clear
D)
\[\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 217) If a coin is tossed n times, then the probability that head comes odd number of times is
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{{{2}^{n}}}\]
done
clear
C)
\[\frac{1}{{{2}^{n-1}}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 218) \[\int{\sqrt{1+\sec x}}dx\]is equal to
A)
\[2{{\sin }^{-1}}(\sqrt{2}\sin x)+c\]
done
clear
B)
\[-2{{\sin }^{-1}}\left( \sqrt{2}\sin \frac{x}{2} \right)+c\]
done
clear
C)
\[2{{\sin }^{-1}}\left( \sqrt{2}\sin \frac{x}{2} \right)+c\]
done
clear
D)
\[\frac{1}{2}{{\sin }^{-1}}(\sqrt{2}\sin x)+c\]
done
clear
View Answer play_arrow
question_answer 219) \[\int{\cos (\log x)dx}\]is equal to
A)
\[\frac{x}{2}[\sin (\log x)-\cos (\log x)]+c\]
done
clear
B)
\[\frac{x}{2}[\sin (\log x)+\cos (\log x)]+c\]
done
clear
C)
\[\frac{1}{2}[\sin (\log x)-\cos (\log x)]+c\]
done
clear
D)
\[\frac{1}{2}[\sin (\log x)+\cos (\log x)]+c\]
done
clear
View Answer play_arrow
question_answer 220) The value of\[|{{z}_{1}}+{{z}_{2}}{{|}^{2}}+|{{z}_{1}}-{{z}_{2}}{{|}^{2}}\]is
A)
\[\frac{1}{2}[|{{z}_{1}}{{|}^{2}}+|{{z}_{2}}{{|}^{2}}]\]
done
clear
B)
\[2[|{{z}_{1}}{{|}^{2}}+|{{z}_{2}}{{|}^{2}}]\]
done
clear
C)
\[2[|{{z}_{1}}{{|}^{2}}-|{{z}_{2}}{{|}^{2}}]\]
done
clear
D)
\[\frac{1}{2}[|{{z}_{1}}{{|}^{2}}-|{{z}_{2}}{{|}^{2}}]\]
done
clear
View Answer play_arrow
question_answer 221) If 5th term and 11th term of a harmonic progression are\[\frac{1}{45}\]and\[\frac{1}{69}\]respectively, then 16th term is
A)
\[\frac{1}{89}\]
done
clear
B)
\[\frac{1}{85}\]
done
clear
C)
\[\frac{1}{81}\]
done
clear
D)
\[\frac{1}{77}\]
done
clear
View Answer play_arrow
question_answer 222) The geometric mean of the roots of the equation\[{{x}^{2}}-18x+9=0\]is
A)
\[9\sqrt{2}\]
done
clear
B)
\[9\]
done
clear
C)
\[\frac{1}{81}\]
done
clear
D)
\[\frac{1}{77}\]
done
clear
View Answer play_arrow
question_answer 223) If the roots of the equation \[{{x}^{2}}-8x+{{a}^{2}}-60=0\]are real, then the value of\[a\]is
A)
\[-2<a<8\]
done
clear
B)
\[-2<a<8\]
done
clear
C)
\[-2<a<8\]
done
clear
D)
\[2<a<8\]
done
clear
View Answer play_arrow
question_answer 224) The equation of common tangent to the circle \[{{x}^{2}}+{{y}^{2}}=2\]and the parabola\[{{y}^{2}}=8x\]is
A)
\[y=x+1\]
done
clear
B)
\[y=x+2\]
done
clear
C)
\[y=x-2\]
done
clear
D)
\[y=-x+2\]
done
clear
View Answer play_arrow
question_answer 225) The angle between the tangents drawn from the origin to the circle\[{{(x-7)}^{2}}+{{(y+1)}^{2}}=25\]is
A)
\[\frac{\pi }{3}\]
done
clear
B)
\[\frac{\pi }{6}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\frac{\pi }{8}\]
done
clear
View Answer play_arrow
question_answer 226) \[{{\left( \frac{\sqrt{3}+i}{2} \right)}^{6}}+{{\left( \frac{i-\sqrt{3}}{2} \right)}^{6}}\]is equal to
A)
0
done
clear
B)
\[-1\]
done
clear
C)
2
done
clear
D)
\[-2\]
done
clear
View Answer play_arrow
question_answer 227) If\[A=\left[ \begin{matrix} 3 & 2 \\ 1 & -4 \\ \end{matrix} \right],\]then A (adj A) is equal to
A)
\[-14I\]
done
clear
B)
\[-10I\]
done
clear
C)
\[8I\]
done
clear
D)
\[-\frac{1}{14}I\]
done
clear
View Answer play_arrow
question_answer 228) Maximum value of\[\frac{\log x}{x}\]is.
A)
e
done
clear
B)
1
done
clear
C)
2e
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 229) If the line\[lx+my=1\]is the tangent to the circle\[{{x}^{2}}+{{y}^{2}}={{r}^{2}},\]then locus of\[(l,m)\]is
A)
\[{{x}^{2}}+{{y}^{2}}=1\]
done
clear
B)
\[{{r}^{2}}({{x}^{2}}+{{y}^{2}})=1\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}={{r}^{2}}\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}=2{{r}^{2}}\]
done
clear
View Answer play_arrow
question_answer 230) \[\frac{d}{dx}{{\cosh }^{-1}}(\sec x)\]is equal to
A)
\[sin\text{ }x\]
done
clear
B)
\[sec\text{ }x\]
done
clear
C)
\[tan\text{ }x\]
done
clear
D)
\[cosec\text{ }x\]
done
clear
View Answer play_arrow
question_answer 231) If\[\overrightarrow{a}=2\hat{i}-\hat{j}+\hat{k},\overrightarrow{b}=\hat{j}+\hat{k}\]and\[\overrightarrow{c}=\hat{i}-\hat{k}\]and \[\overrightarrow{a}+\overrightarrow{b}\]and\[\overrightarrow{b}+\overrightarrow{c}\]are the diagonals of a parallelogram, then it's vector area is equal to
A)
\[\hat{i}+\hat{j}+\hat{k}\]
done
clear
B)
\[-\hat{i}+\hat{j}+\hat{k}\]
done
clear
C)
\[\hat{i}-\hat{j}+\hat{k}\]
done
clear
D)
\[\hat{i}+\hat{j}-\hat{k}\]
done
clear
View Answer play_arrow
question_answer 232) If\[\overrightarrow{a}=\overrightarrow{b}+\overrightarrow{c},\]then\[\overrightarrow{a}(\overrightarrow{b}\times \overrightarrow{c})\]is equal to
A)
\[\overrightarrow{a}.(\overrightarrow{b}+\overrightarrow{c})\]
done
clear
B)
\[2\overrightarrow{a}.(\overrightarrow{b}+\overrightarrow{c})\]
done
clear
C)
\[\overrightarrow{b}.(\overrightarrow{a}+\overrightarrow{c})\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 233) \[\int_{0}^{1}{\sqrt{\frac{1-x}{1+x}}}dx\]is equal to
A)
\[\frac{\pi }{2}-1\]
done
clear
B)
\[\frac{\pi }{2}+1\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\pi +1\]
done
clear
View Answer play_arrow
question_answer 234) If\[\alpha \]and\[\beta \]are the roots of the equationx\[{{x}^{2}}-(1+{{n}^{2}})x+\frac{1}{2}(1+{{n}^{2}}+{{n}^{4}})=0\]then\[{{\alpha }^{2}}+{{\beta }^{2}}\]is equal to
A)
\[{{n}^{2}}+2\]
done
clear
B)
\[-{{n}^{2}}\]
done
clear
C)
\[{{n}^{2}}\]
done
clear
D)
\[2{{n}^{2}}\]
done
clear
View Answer play_arrow
question_answer 235) If\[{{(1+x)}^{n}}{{=}^{n}}{{C}_{0}}{{+}^{n}}{{C}_{1}}x{{+}^{n}}{{C}_{2}}{{x}^{2}}+....{{+}^{n}}{{C}_{n}}{{x}^{n}},\]then the value of \[\frac{^{n}{{C}_{1}}}{^{n}{{C}_{0}}}+2.\frac{^{n}{{C}_{2}}}{^{n}{{C}_{1}}}+3.\frac{^{n}{{C}_{3}}}{^{n}{{C}_{2}}}+....+n.\frac{^{n}{{C}_{n}}}{^{n}{{C}_{n-1}}}\]is
A)
\[\frac{n(n+1)}{2}\]
done
clear
B)
\[\frac{(n+1)}{n!}\]
done
clear
C)
\[\frac{n({{n}^{2}}+1)}{2}\]
done
clear
D)
\[n\frac{(n+1)}{2}\]
done
clear
View Answer play_arrow
question_answer 236) \[\frac{1-2i}{2+i}+\frac{4-i}{3+2i}\]is equal to
A)
\[-\frac{24}{13}+\frac{10}{13}i\]
done
clear
B)
\[\frac{24}{13}-\frac{10}{13}i\]
done
clear
C)
\[\frac{10}{13}-\frac{24}{13}i\]
done
clear
D)
\[\frac{10}{13}+\frac{24}{13}i\]
done
clear
View Answer play_arrow
question_answer 237) If\[\omega \]is the cube root of unity, then the value of \[\left| \begin{matrix} 1 & \omega & {{\omega }^{2}} \\ \omega & {{\omega }^{2}} & 1 \\ {{\omega }^{2}} & 1 & \omega \\ \end{matrix} \right|\]is equal to
A)
1
done
clear
B)
0
done
clear
C)
\[\omega \]
done
clear
D)
\[{{\omega }^{2}}+1\]
done
clear
View Answer play_arrow
question_answer 238) If \[z=\frac{(1-i)(2+i)}{3+i},\]then\[|z|\]is equal to
A)
\[-1\]
done
clear
B)
1
done
clear
C)
\[1/2\]
done
clear
D)
\[-1/2\]
done
clear
View Answer play_arrow
question_answer 239) In the expansion of\[{{\left( 2{{x}^{4}}-\frac{1}{{{x}^{7}}} \right)}^{11}},\]the term independent of \[x\]is
A)
330
done
clear
B)
42240
done
clear
C)
114050
done
clear
D)
\[-32190\]
done
clear
View Answer play_arrow
question_answer 240) The equation of radical axis of the circles \[2{{x}^{2}}+2{{y}^{2}}-7x=0\]and\[{{x}^{2}}+{{y}^{2}}-4y-7=0\]is
A)
\[7x+8y+14=0\]
done
clear
B)
\[7x-8y-14=0\]
done
clear
C)
\[7x-8y+14=0\]
done
clear
D)
\[8x-7y+14=0\]
done
clear
View Answer play_arrow
question_answer 241) If\[f(\theta )=\tan \theta ,\]then the value of\[\frac{f(\theta )-f(\phi )}{1+f(\theta )f(\phi )}\]is
A)
\[f(\theta )+f(\phi )\]
done
clear
B)
\[f(\theta -\phi )\]
done
clear
C)
\[f\left( \frac{\theta }{\phi } \right)\]
done
clear
D)
\[\theta -\phi \]
done
clear
View Answer play_arrow
question_answer 242) If\[A=\left[ \begin{matrix} 2 & 14 \\ 0 & 3 \\ \end{matrix} \right]\]and\[B=\left[ \begin{matrix} 1 & 2 \\ 0 & 5 \\ \end{matrix} \right],\]then\[4A-3B\]is equal to
A)
\[\left[ \begin{matrix} 7 & 14 \\ 0 & 7 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 5 & 10 \\ 0 & -3 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} -5 & -10 \\ 0 & 3 \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} 1 & 2 \\ 0 & 2 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 243) If two vertices of a triangle are (6, 4) and (2,6) and centroid is (4,6), then the third vertex is
A)
(4, 8)
done
clear
B)
(8, 4)
done
clear
C)
(6, 4)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 244) \[\frac{d}{dx}({{\sec }^{-1}}x)\]is equal to
A)
\[\frac{1}{x\sqrt{1-{{x}^{2}}}}\]
done
clear
B)
\[\frac{1}{x\sqrt{1+x}}\]
done
clear
C)
\[\frac{1}{x\sqrt{{{x}^{2}}-1}}\]
done
clear
D)
\[\frac{1}{x\sqrt{x-1}}\]
done
clear
View Answer play_arrow
question_answer 245) Differential coefficient of\[{{\sin }^{-1}}x\]with respect to \[{{\cos }^{-1}}\sqrt{1-{{x}^{2}}}\]is
A)
\[\frac{2}{\sqrt{1-{{x}^{2}}}}\]
done
clear
B)
\[\frac{1}{\sqrt{1-{{x}^{2}}}}\]
done
clear
C)
\[-\frac{1}{\sqrt{1+{{x}^{2}}}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 246) \[\int{{{\cos }^{3}}xdx}\]is equal to
A)
\[{{\sin }^{3}}x+c\]
done
clear
B)
\[\frac{\sin 3x}{3}+\frac{\sin x}{2}+c\]
done
clear
C)
\[\frac{\sin 3x}{12}+\frac{3}{4}\sin x+c\]
done
clear
D)
\[\frac{\sin 3x}{4}+3\sin x+c\]
done
clear
View Answer play_arrow
question_answer 247) \[\frac{d}{dx}{{(\sin x)}^{\tan x}}\]is equal to
A)
\[{{(\sin x)}^{\tan x}}[1+{{\sec }^{2}}x\log \sin x]\]
done
clear
B)
\[{{(\sin x)}^{\tan x}}[1-{{\sec }^{2}}x\log \sin x]\]
done
clear
C)
\[{{(\tan x)}^{\sin x}}[\log {{\sec }^{2}}x\log \sin x]\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 248) When two dices are thrown together, then the probability that sum of the digits is seven, is
A)
\[\frac{5}{36}\]
done
clear
B)
\[\frac{1}{12}\]
done
clear
C)
\[\frac{1}{6}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 249) A card is drawn from a pack of cards. The probability that this card is diamond or ace, is
A)
\[\frac{4}{13}\]
done
clear
B)
\[\frac{17}{52}\]
done
clear
C)
\[\frac{1}{52}\]
done
clear
D)
\[\frac{1}{13}\]
done
clear
View Answer play_arrow
question_answer 250) The value of the determinant\[\left| \begin{matrix} a+ib & c+ib \\ c-id & a-ib \\ \end{matrix} \right|\]is
A)
\[(a+b)(c-d)\]
done
clear
B)
\[({{a}^{2}}+{{b}^{2}})({{c}^{2}}-{{d}^{2}})\]
done
clear
C)
\[{{a}^{2}}+{{b}^{2}}-{{c}^{2}}-{{d}^{2}}\]
done
clear
D)
\[{{a}^{2}}-{{b}^{2}}+{{c}^{2}}-{{d}^{2}}\]
done
clear
View Answer play_arrow
question_answer 251) The value of\[\left| \begin{matrix} 43 & 1 & 6 \\ 35 & 7 & 4 \\ 17 & 3 & 2 \\ \end{matrix} \right|\]is
A)
150
done
clear
B)
0
done
clear
C)
\[-110\]
done
clear
D)
\[-6\]
done
clear
View Answer play_arrow
question_answer 252) If roots of the equation\[{{x}^{2}}-ax+b=0\]are a and\[\beta \]and\[{{V}_{n}}={{\alpha }^{n}}+{{\beta }^{n}},\]then
A)
\[{{V}_{n+1}}=\alpha {{V}_{n}}-b{{V}_{n-1}}\]
done
clear
B)
\[{{V}_{n+1}}=b{{V}_{n}}+a{{V}_{n-1}}\]
done
clear
C)
\[{{V}_{n+1}}=b{{V}_{n}}-a{{V}_{n-1}}\]
done
clear
D)
\[{{V}_{n+1}}=b{{V}_{n}}+b{{V}_{n-1}}\]
done
clear
View Answer play_arrow
question_answer 253) If arithmetic mean and harmonic mean of two numbers be 27 and 12, then their geometric mean is equal to
A)
36
done
clear
B)
24
done
clear
C)
18
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 254) Real part of\[(\alpha +i\beta )\]is
A)
\[cosh\text{ }\alpha \text{ }cos\text{ }\beta \]
done
clear
B)
\[cos\text{ }\alpha \text{ }cos\text{ }\beta \]
done
clear
C)
\[cos\text{ }\alpha \text{ }cosh\text{ }\beta \]
done
clear
D)
\[sin\text{ }\alpha \text{ }sinh\text{ }\beta \]
done
clear
View Answer play_arrow
question_answer 255) If A be a square matrix and\[A+{{A}^{T}}\]is a symmetric matrix, then\[A-{{A}^{T}}\]is
A)
a unit matrix
done
clear
B)
a symmetric matrix
done
clear
C)
a skew-symmetric matrix
done
clear
D)
a zero matrix
done
clear
View Answer play_arrow
question_answer 256) If\[\alpha \]and\[\beta \]be the roots of the equation \[{{x}^{2}}-2x+4=0,\]then the value of\[{{\alpha }^{n}}+{{\beta }^{n}}\]is
A)
\[{{2}^{n-1}}\cos \left( \frac{n\pi }{3} \right)\]
done
clear
B)
\[i{{2}^{n-1}}\sin \left( \frac{n\pi }{3} \right)\]
done
clear
C)
\[{{2}^{n+1}}\cos \left( \frac{n\pi }{3} \right)\]
done
clear
D)
\[i{{2}^{n+1}}\sin \left( \frac{n\pi }{3} \right)\]
done
clear
View Answer play_arrow
question_answer 257) If the normal to any curve is parallel to\[x-\]axis, then the true statement is
A)
\[\frac{dy}{dx}=0\]
done
clear
B)
\[\frac{dx}{dy}=0\]
done
clear
C)
\[\frac{dy}{dx}=1\]
done
clear
D)
\[\frac{dx}{dy}=-1\]
done
clear
View Answer play_arrow
question_answer 258) Differential coefficient of\[{{x}^{3}}\]with respect to\[{{x}^{2}}\]
A)
\[\frac{3{{x}^{2}}}{2}\]
done
clear
B)
\[\frac{3x}{2}\]
done
clear
C)
\[\frac{3}{2}x\]
done
clear
D)
\[\frac{3}{2x}\]
done
clear
View Answer play_arrow
question_answer 259) If A and B are two events, then\[P\left( \frac{\overline{A}}{B} \right)\]is equal to
A)
\[1-P\left( \frac{A}{B} \right)\]
done
clear
B)
\[\frac{1-P(AB)}{P(B)}\]
done
clear
C)
\[\frac{1-P(A+B)}{P(\overline{B})}\]
done
clear
D)
\[\frac{P(\overline{A})}{P(\overline{B})}\]
done
clear
View Answer play_arrow
question_answer 260) \[\overrightarrow{a}\times (\overrightarrow{b}\times \overrightarrow{c})\]is equal to
A)
\[(\overrightarrow{a}.\overrightarrow{b})\overrightarrow{c}+(\overrightarrow{a}.\overrightarrow{c})\overrightarrow{b}\]
done
clear
B)
\[(\overrightarrow{a}.\overrightarrow{b})\overrightarrow{c}-(\overrightarrow{a}.\overrightarrow{c})\overrightarrow{b}\]
done
clear
C)
\[(\overrightarrow{a}.\overrightarrow{c})\overrightarrow{b}-(\overrightarrow{a}.\overrightarrow{b})\overrightarrow{c}\]
done
clear
D)
\[(\overrightarrow{a}.\overrightarrow{c})\overrightarrow{b}+(\overrightarrow{a}.\overrightarrow{b})\overrightarrow{c}\]
done
clear
View Answer play_arrow
question_answer 261) For any two events A and B, which of the following is true?
A)
\[P(A\cap B)\ge P(A)+P(B)-1\]
done
clear
B)
\[P(A\cap B)\le P(A)+P(B)-1\]
done
clear
C)
\[P(A\cap B)\ge P(A)+P(B)\]
done
clear
D)
\[P(A\cap B)\le P(A)+P(B)\]
done
clear
View Answer play_arrow
question_answer 262) \[P\left( \frac{\overline{A}}{A\cup B} \right)\]is equal to
A)
\[\frac{P(\overline{B})}{P(A\cup B)}\]
done
clear
B)
\[\frac{P(\overline{A})}{P(A\cup B)}\]
done
clear
C)
\[\frac{P(\overline{A}\cap B)}{P(A\cup B)}\]
done
clear
D)
\[\frac{P(A)}{P(A\cup B)}\]
done
clear
View Answer play_arrow
question_answer 263) If the vectors\[2\hat{i}+\hat{j}-\hat{k}\]and\[\hat{i}-4\hat{j}+\lambda \hat{k}\]are perpendicular to each other, then\[\lambda \]is equal to
A)
0
done
clear
B)
\[-1\]
done
clear
C)
\[-2\]
done
clear
D)
\[-3\]
done
clear
View Answer play_arrow
question_answer 264) If\[f(a-x)=f(x),\]then\[\int_{0}^{a}{x}f(x)dx\]is equal to
A)
\[a\int_{0}^{a}{f(x)dx}\]
done
clear
B)
\[\frac{a}{2}\int_{0}^{a}{f(x)dx}\]
done
clear
C)
\[\int_{0}^{a}{f(x)dx}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 265) If\[A=\left[ \begin{matrix} i & 0 \\ 0 & i \\ \end{matrix} \right]\]and\[B=\left[ \begin{matrix} 0 & -i \\ -i & 0 \\ \end{matrix} \right],\]then \[(A+B)(A-B)\]is equal to
A)
2A
done
clear
B)
2B
done
clear
C)
\[2I\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 266) If\[{{x}^{y}}={{e}^{x-y}},\]then\[\frac{dy}{dx}\] is equal to
A)
\[\frac{\log x}{{{(1+\log x)}^{2}}}\]
done
clear
B)
\[\frac{y-x}{x(1+\log x)}\]
done
clear
C)
\[\frac{x-y}{1+\log \,\,x}\]
done
clear
D)
\[\frac{y-x}{x(1+log\,\,x)}\]
done
clear
View Answer play_arrow
question_answer 267) If intercept on made by a line on the axes are a and b and its distance from the origin is p, then
A)
\[\frac{1}{{{p}^{2}}}={{a}^{2}}+{{b}^{2}}\]
done
clear
B)
\[{{p}^{2}}={{a}^{2}}+{{b}^{2}}\]
done
clear
C)
\[{{p}^{2}}=\frac{1}{{{a}^{2}}}+\frac{1}{{{b}^{2}}}\]
done
clear
D)
\[\frac{1}{{{p}^{2}}}=\frac{1}{{{a}^{2}}}+\frac{1}{{{b}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 268) If the circle\[{{x}^{2}}+{{y}^{2}}+2ax+8y+16=0\]touches the\[x-\]axis, then a is equal to
A)
±16
done
clear
B)
± 8
done
clear
C)
± 1
done
clear
D)
± 4
done
clear
View Answer play_arrow
question_answer 269) The true statement is
A)
\[cos{{h}^{2}}x+sin{{h}^{2}}x=1\]
done
clear
B)
\[cos{{h}^{2}}x-sin{{h}^{2}}x=cos{{h}^{2}}x\]
done
clear
C)
\[1+tan{{h}^{2}}x=sec{{h}^{2}}x\]
done
clear
D)
\[cos{{h}^{2}}x-sin{{h}^{2}}x=1\]
done
clear
View Answer play_arrow
question_answer 270) If the points (1,2),\[(-1,x)\]and (2,3) are collinear, then\[x\]is equal to
A)
1
done
clear
B)
2
done
clear
C)
0
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 271) A hits an aim four times out of 5 trials, B hits three times out of 4 trials and C hits two times out of 3 trials. The probability that only two hits the target is
A)
\[\frac{5}{12}\]
done
clear
B)
\[\frac{5}{6}\]
done
clear
C)
\[\frac{25}{60}\]
done
clear
D)
\[\frac{26}{60}\]
done
clear
View Answer play_arrow
question_answer 272) A card is drawn from a pack of cards. The probability that this card is an ace or a queen is
A)
\[\frac{4}{13}\]
done
clear
B)
\[\frac{17}{52}\]
done
clear
C)
\[\frac{3}{13}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 273) The probability of occuring 1 or 6 when a dice is thrown, is
A)
\[\frac{1}{6}\]
done
clear
B)
\[\frac{1}{36}\]
done
clear
C)
\[\frac{2}{3}\]
done
clear
D)
\[\frac{1}{3}\]
done
clear
View Answer play_arrow
question_answer 274) Which one is not true from the following?
A)
\[{{(A+B)}^{T}}={{A}^{T}}+{{B}^{T}}\]
done
clear
B)
\[{{({{A}^{T}})}^{T}}=A\]
done
clear
C)
\[{{(KA)}^{T}}=K{{A}^{T}}\]
done
clear
D)
\[{{(AB)}^{T}}={{A}^{T}}{{B}^{T}}\]
done
clear
View Answer play_arrow
question_answer 275) If\[{{z}_{1}}\]and\[{{z}_{2}}\]are any two complex numbers, then true relation is
A)
\[|{{z}_{1}}+{{z}_{2}}|\le |{{z}_{1}}|-|{{z}_{2}}|\]
done
clear
B)
\[|{{z}_{1}}-{{z}_{2}}|\ge |{{z}_{1}}|+|{{z}_{2}}|\]
done
clear
C)
\[|{{z}_{1}}+{{z}_{2}}|\ge |{{z}_{1}}|+|{{z}_{2}}|\]
done
clear
D)
\[|{{z}_{1}}-{{z}_{2}}|\le |{{z}_{1}}|+|{{z}_{2}}|\]
done
clear
View Answer play_arrow
question_answer 276) The equation of parabola which has the vertex at origin and axis is\[x-\]axis and also passes through the point (4,3), is
A)
\[9{{y}^{2}}=4x\]
done
clear
B)
\[9{{y}^{2}}=-4x\]
done
clear
C)
\[4{{y}^{2}}=-9x\]
done
clear
D)
\[4{{y}^{2}}=9x\]
done
clear
View Answer play_arrow
question_answer 277) If\[3{{y}^{2}}-4{{x}^{2}}=0,\]then\[\frac{{{d}^{2}}y}{d{{x}^{2}}}\]is equal to
A)
\[\frac{{{y}^{3}}}{2{{y}^{2}}-4{{x}^{2}}}\]
done
clear
B)
\[\frac{2{{y}^{2}}-4{{x}^{2}}}{{{y}^{3}}}\]
done
clear
C)
\[\frac{4y-8x}{{{y}^{3}}}\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 278) The equation of that circle which passes through the origin and cut the axes\[x\]and y of length 6 and 8 on respectively, is
A)
\[{{x}^{2}}+{{y}^{2}}\pm 6x\pm 8y=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}\pm 8x\pm 6y=0\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}\pm 12x\pm 16y=0\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}\pm 16x\pm 12y=0\]
done
clear
View Answer play_arrow
question_answer 279) The equation of directrix of the parabola \[{{y}^{2}}=-12x\]is
A)
\[x+3=0\]
done
clear
B)
\[x=3\]
done
clear
C)
\[y+3=0\]
done
clear
D)
\[y-3=0\]
done
clear
View Answer play_arrow
question_answer 280) \[\int{\frac{x\,dx}{1+\cos x}}\]is equal to
A)
\[x\tan \frac{x}{2}+2\log \cos \frac{x}{2}+c\]
done
clear
B)
\[x\tan \frac{x}{2}-\log \cos \frac{x}{2}+c\]
done
clear
C)
\[2\left\{ x\tan \frac{x}{2}+\log \cos \frac{x}{2} \right\}+c\]
done
clear
D)
\[x\tan \frac{x}{2}-\log \cos \frac{x}{2}+c\]
done
clear
View Answer play_arrow
question_answer 281) If\[x=a\text{ }cos\text{ }t,\text{ }y=b\text{ }sin\text{ }t,\]then\[\frac{dy}{dx}\]is equal to
A)
\[-\frac{b}{a}\tan t\]
done
clear
B)
\[-\frac{b}{a}\cot t\]
done
clear
C)
\[-\frac{a}{b}\cot t\]
done
clear
D)
\[-\frac{a}{b}\tan t\]
done
clear
View Answer play_arrow
question_answer 282) If\[y=\frac{1}{(t+2)(t+1)},\]then\[\frac{dy}{dx}\]is equal to
A)
\[-\frac{1}{{{(t+1)}^{2}}}+\frac{1}{{{(t+2)}^{2}}}\]
done
clear
B)
\[\frac{1}{{{(t+1)}^{2}}}+\frac{1}{{{(t+2)}^{2}}}\]
done
clear
C)
\[-\frac{1}{{{(t+1)}^{2}}}-\frac{1}{{{(t+2)}^{2}}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 283) The equation of tangents at that point where the curve\[y={{x}^{2}}-3x+2\]cut the\[x-\]axis, is
A)
\[x+y-1=0,x-y-2=0\]
done
clear
B)
\[x-y-1=0,\text{ }x-y=0\]
done
clear
C)
\[x-y+2=0,x-y-1=0\]
done
clear
D)
\[x+y=0,\text{ }x-y=0\]
done
clear
View Answer play_arrow
question_answer 284) \[\int_{0}^{a}{\sqrt{\frac{a}{a-x}}}dx\]is equal to
A)
\[4a\]
done
clear
B)
\[-4a\]
done
clear
C)
\[2a\]
done
clear
D)
\[a\]
done
clear
View Answer play_arrow
question_answer 285) \[\int{\frac{2dx}{{{x}^{2}}-1}}\] is equal to
A)
\[\frac{1}{2}\log \frac{x-1}{x+1}+c\]
done
clear
B)
\[\log \frac{x-1}{x+1}+c\]
done
clear
C)
\[\frac{1}{2}\log \frac{x+1}{x-1}+c\]
done
clear
D)
\[\log \frac{x+1}{x-1}+c\]
done
clear
View Answer play_arrow
question_answer 286) Two cards are drawn one by one from a pack of 52 cards. The probability that these axes, is
A)
\[\frac{1}{26}\]
done
clear
B)
\[\frac{1}{221}\]
done
clear
C)
\[\frac{2}{21}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 287) If there are 4 copper coins and 3 silver coins in a purse while 6 copper coins and 2 silver coins in an another purse a coin is taken randomly from any one of the purse, the probability that this is of copper coin is
A)
\[\frac{4}{7}\]
done
clear
B)
\[\frac{3}{4}\]
done
clear
C)
\[\frac{2}{7}\]
done
clear
D)
\[\frac{37}{56}\]
done
clear
View Answer play_arrow
question_answer 288) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{\tan x-\sin x}{{{x}^{3}}}\]is equal to
A)
\[-1\]
done
clear
B)
\[1/2\]
done
clear
C)
2
done
clear
D)
\[-2\]
done
clear
View Answer play_arrow
question_answer 289) The order of a matrix A is\[P\times Q\]and that of B is\[R\times S,\]then for\[A-B,\]the values of P and Q are
A)
\[P=Q\]
done
clear
B)
\[P=Q,R=S\]
done
clear
C)
\[P=R,\text{ }Q=S\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 290) If\[f(x)=\frac{x}{\sqrt{1+{{x}^{2}}}},\]then\[fofof(x)\]is equal to
A)
\[\frac{x}{\sqrt{1+3{{x}^{2}}}}\]
done
clear
B)
\[\frac{x}{\sqrt{1+{{x}^{2}}}}\]
done
clear
C)
\[\frac{x}{\sqrt{1+2{{x}^{2}}}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 291) Argument of\[\left( \frac{1-i\sqrt{3}}{1+i\sqrt{3}} \right)\]is
A)
\[60{}^\circ \]
done
clear
B)
\[120{}^\circ \]
done
clear
C)
\[-120{}^\circ \]
done
clear
D)
\[-135{}^\circ \]
done
clear
View Answer play_arrow
question_answer 292) If\[^{n-1}{{C}_{3}}{{+}^{n-1}}{{C}_{4}}{{>}^{n}}{{C}_{3}},\]then n is equal to
A)
4
done
clear
B)
6
done
clear
C)
8
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 293) Which one of the following is an even function?
A)
\[f(x)=\frac{{{a}^{x}}+{{a}^{-x}}}{{{a}^{x}}-{{a}^{-x}}}\]
done
clear
B)
\[f(x)=\frac{{{a}^{x}}+1}{{{a}^{x}}-1}\]
done
clear
C)
\[f(x)=x\frac{{{a}^{x}}-1}{{{a}^{x}}+1}\]
done
clear
D)
\[f(x)={{\log }_{e}}(x+\sqrt{{{x}^{2}}+1})\]
done
clear
View Answer play_arrow
question_answer 294) Locus of the centroid of the triangle with vertices\[(a\text{ }cos\text{ }t,a\text{ }sin\text{ }t),(b\text{ }sin\text{ }t,-b\text{ }cos\text{ }t)\] and (1,0), where t is a parameter, is
A)
\[{{(3x-1)}^{2}}+{{(3y)}^{2}}={{a}^{2}}-{{b}^{2}}\]
done
clear
B)
\[{{(3x-1)}^{2}}+{{(3y)}^{2}}={{a}^{2}}+{{b}^{2}}\]
done
clear
C)
\[{{(3x+1)}^{2}}+{{(3y)}^{2}}={{a}^{2}}+{{b}^{2}}\]
done
clear
D)
\[{{(3x+1)}^{2}}+{{(3y)}^{2}}={{a}^{2}}-{{b}^{2}}\]
done
clear
View Answer play_arrow
question_answer 295) If sum of squares of the roots of the equation \[{{x}^{2}}-(a-2)x-(a+1)=0\]is minimum, then the value of a is
A)
0
done
clear
B)
2
done
clear
C)
\[-1\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 296) If\[{{A}^{T}}\]is the transpose matrix of the matrix A, then \[A{{A}^{T}}\]is
A)
symmetric matrix
done
clear
B)
inverse matrix
done
clear
C)
skew-symmetric matrix
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 297) If\[y=f(x)=\frac{ax+b}{cx-a},\]then\[x\]is equal to
A)
\[\frac{1}{f(x)}\]
done
clear
B)
\[\frac{1}{f(y)}\]
done
clear
C)
\[yf(x)\]
done
clear
D)
\[f(y)\]
done
clear
View Answer play_arrow
question_answer 298) Domain of the function\[f(x)=\sqrt{2-2x-{{x}^{2}}}\]is
A)
\[-\sqrt{3}\le x\le \sqrt{3}\]
done
clear
B)
\[-1-\sqrt{3}\le x\le -1+\sqrt{3}\]
done
clear
C)
\[-2\le x\le 2\]
done
clear
D)
\[-2+\sqrt{3}\le x\le -2-\sqrt{3}\]
done
clear
View Answer play_arrow
question_answer 299) Equation of tangent to the ellipse \[{{x}^{2}}+16{{y}^{2}}=16\]which makes an angle of\[60{}^\circ \]with\[x-\]axis, is
A)
\[\sqrt{3}x-y+7=0\]
done
clear
B)
\[\sqrt{3}x-y-7=0\]
done
clear
C)
\[\sqrt{3}x-y\pm 7=0\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 300) Intersection points of the circles\[{{x}^{2}}+{{y}^{2}}=25\] and \[{{x}^{2}}+{{y}^{2}}-8x+7=0,\]are
A)
(4, 3) and \[(4,-3)\]
done
clear
B)
\[(4,-3)\]and\[(-4,-3)\]
done
clear
C)
\[(-4,3)\]and (4, 3)
done
clear
D)
(4, 3) and (3, 4)
done
clear
View Answer play_arrow
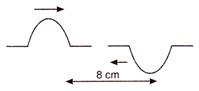 After 2s, the total energy of the pulses will be
After 2s, the total energy of the pulses will be