question_answer 1) Which of the following equation represents a progressive wave?
A)
\[y=\,A\cos \,ax\,\sin bt\]
done
clear
B)
\[y=\,A\,\sin \,bt\]
done
clear
C)
\[y=\,A\,\cos (ax+bt)\]
done
clear
D)
\[y=\,A\,\tan (ax+bt)\]
done
clear
View Answer play_arrow
question_answer 2) The frequency and velocity of sound wave are 600 Hz and 360 m/s respectively. Phase difference between two particles of medium are\[60{}^\circ ,\]the minimum distance between these two particles will be
A)
10 cm
done
clear
B)
15 cm
done
clear
C)
20 cm
done
clear
D)
50 cm
done
clear
View Answer play_arrow
question_answer 3) The heat required to increase the temperature of 4 moles of a monoatomic ideal gas from 273 K to 473 K at constant volume is
A)
200 H
done
clear
B)
400 R
done
clear
C)
800 J
done
clear
D)
1200 R
done
clear
View Answer play_arrow
question_answer 4) A solid sphere rolls without slipping on the roof. The ratio of its rotational kinetic energy and its total kinetic energy is
A)
2/5
done
clear
B)
4/5
done
clear
C)
2/7
done
clear
D)
3/7
done
clear
View Answer play_arrow
question_answer 5) A black body at a temperature of 2600 K has the wavelength corresponding to maximum emission 1200\[\overset{o}{\mathop{\text{A}}}\,\]. Assuming the moon to be perfectly black body the temperature of the moon, if the wavelength corresponding to maximum emission is 5000\[\overset{o}{\mathop{\text{A}}}\,\] is
A)
7800 K
done
clear
B)
6240 K
done
clear
C)
5240 K
done
clear
D)
3640 K
done
clear
View Answer play_arrow
question_answer 6) When the light enters from air to glass, for which colour the angle of deviation is maximum?
A)
Red
done
clear
B)
Yellow
done
clear
C)
Blue
done
clear
D)
Violet
done
clear
View Answer play_arrow
question_answer 7) Two waves are represent by \[{{y}_{1}}=A\sin (kx-\omega t)\,and\,{{y}_{2}}=A\cos \,(kx-\omega t)\] The amplitude of resultant wave is
A)
4A
done
clear
B)
2A
done
clear
C)
\[\sqrt{2}A\]
done
clear
D)
A
done
clear
View Answer play_arrow
question_answer 8) Which of the following event, support the quantum nature of light?
A)
Diffraction
done
clear
B)
Polarization
done
clear
C)
Interference
done
clear
D)
Photoelectric effect
done
clear
View Answer play_arrow
question_answer 9) The linear momentum of photon is p. The wavelength of photon is \[\lambda \], then (\[h\]is Planck?s constant)
A)
\[\lambda =hp\]
done
clear
B)
\[\lambda =\frac{h}{p}\]
done
clear
C)
\[\lambda =\frac{p}{h}\]
done
clear
D)
\[\lambda =\frac{{{p}^{2}}}{h}\]
done
clear
View Answer play_arrow
question_answer 10) When two coherent monochromatic beams of intensity I and 9l interfere, the possible maximum and minimum intensities of the resulting beam are
A)
\[9I\] and\[I\]
done
clear
B)
\[9I\]and\[4I\]
done
clear
C)
\[16I\]and\[4I\]
done
clear
D)
\[16I\]and\[I\]
done
clear
View Answer play_arrow
question_answer 11) An atom of mass M which is in the state of rest emits a photon of wavelength \[\lambda \] . As a result, the atom will deflect with the kinetic energy equal to (A is Planck's constant)
A)
\[\frac{{{h}^{2}}}{M{{\lambda }^{2}}}\]
done
clear
B)
\[\frac{1}{2}\frac{{{h}^{2}}}{M{{\lambda }^{2}}}\]
done
clear
C)
\[\frac{h}{M\lambda }\]
done
clear
D)
\[\frac{1}{2}\frac{h}{M\lambda }\]
done
clear
View Answer play_arrow
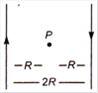
question_answer 12)
Two long straight wires are set parallel to each other Each carries a current i in the opposite direction and the separation between them is 2R. The intensity of the magnetic field midway between them is
A)
zero
done
clear
B)
\[\frac{{{\mu }_{0}}i}{4\pi R}\]
done
clear
C)
\[\frac{{{\mu }_{0}}i}{2\pi R}\]
done
clear
D)
\[\frac{{{\mu }_{0}}i}{\pi R}\]
done
clear
View Answer play_arrow
question_answer 13) Charge Q is placed at the diagonal faced comers of a square and charge q is placed at another two corners of square. The condition for net electric force on Q to be zero will be
A)
\[Q=(-2\sqrt{2)}\,q\]
done
clear
B)
\[Q=-\frac{q}{2}\]
done
clear
C)
\[Q=(2\sqrt{2)}\,q\]
done
clear
D)
\[Q=-\frac{q}{2}\]
done
clear
View Answer play_arrow
question_answer 14) The electric potential at centre of metallic conducting sphere is
A)
zero
done
clear
B)
half from potential at surface of sphere
done
clear
C)
equal from potential at surface of sphere
done
clear
D)
twice from potential at surface of sphere
done
clear
View Answer play_arrow
question_answer 15) A capacitor of capacity 10 \[\mu \]F is charged to a potential of 400 Volt. When its both plates are connected by a conducting wire, then heat generated will be
A)
\[80\text{ }J\]
done
clear
B)
\[0.8\text{ }J\]
done
clear
C)
\[8\times {{10}^{-3}}\]
done
clear
D)
\[8\times {{10}^{-6}}J\]
done
clear
View Answer play_arrow
question_answer 16) Magnet moment of bar magnet is M. The work done to turn the magnet by\[90{}^\circ \]of magnet in direction of magnetic field B will be
A)
zero
done
clear
B)
\[\frac{1}{2}\]MB
done
clear
C)
2 MB
done
clear
D)
MB
done
clear
View Answer play_arrow
question_answer 17) Voltage V and current\[i\]in AC circuit are given by \[\begin{align} & V=50\sin (50\,t)\,volt \\ & i=50\,\sin \,\left( 50t+\frac{\pi }{3} \right)mA \\ \end{align}\] The power dissipated in circuit is
A)
5.0 W
done
clear
B)
2.5 W
done
clear
C)
1.25 W
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 18) The maximum voltage in DC circuit is 282 Volt. The effective voltage in AC circuit will be
A)
200 V
done
clear
B)
300 V
done
clear
C)
400 V
done
clear
D)
564 V
done
clear
View Answer play_arrow
question_answer 19) If L, C and R denote inductance, capacitance and resistance respectively, then which of the following combination has the dimension of time?
A)
\[\frac{C}{L}\]
done
clear
B)
\[\frac{1}{RC}\]
done
clear
C)
\[\frac{L}{R}\]
done
clear
D)
\[\frac{RL}{C}\]
done
clear
View Answer play_arrow
question_answer 20) The ratio of momenta of an electron and photon which are accelerated from rest by a potential difference 50 Volt is
A)
\[\frac{{{m}_{e}}}{{{m}_{p}}}\]
done
clear
B)
\[\sqrt{\frac{{{m}_{e}}}{{{m}_{p}}}}\]
done
clear
C)
\[\frac{{{m}_{p}}}{{{m}_{e}}}\]
done
clear
D)
\[\sqrt{\frac{{{m}_{p}}}{{{m}_{e}}}}\]
done
clear
View Answer play_arrow
question_answer 21) A wire of resistance\[18\,\Omega \]is divided into three equal parts. These parts are connected in side of triangle, the equivalent resistance of any two corners of triangle will be
A)
18\[\Omega \]
done
clear
B)
9\[\Omega \]
done
clear
C)
6\[\Omega \]
done
clear
D)
4\[\Omega \]
done
clear
View Answer play_arrow
question_answer 22) Two capacitors of capacity 6 \[\mu \]F and 12 \[\mu \]V in series are connected by potential of 150 V. The potential of capacitor of capacity 12 \[\mu \]F will be
A)
25 V
done
clear
B)
50 V
done
clear
C)
100 V
done
clear
D)
150 V
done
clear
View Answer play_arrow
question_answer 23) A charged particle enters in a magnetic field whose direction is parallel to velocity of the particle, then the speed of this particle
A)
in straight line
done
clear
B)
in coiled path
done
clear
C)
in circular path
done
clear
D)
in ellipse path
done
clear
View Answer play_arrow
question_answer 24) Magnetic intensity for an axial point due to a short bar magnet of magnetic moment M is given by
A)
\[\frac{{{\mu }_{0}}}{4\pi }\frac{m}{{{d}^{3}}}\]
done
clear
B)
\[\frac{{{\mu }_{0}}M}{4\pi {{d}^{2}}}\]
done
clear
C)
\[\frac{{{\mu }_{0}}}{2\pi }\frac{\mu }{{{d}^{3}}}\]
done
clear
D)
\[\frac{{{\mu }_{0}}M}{2\pi {{d}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 25) Which of the following transition gives the photon of minimum frequency?
A)
n = 2 to n = 1
done
clear
B)
n = 3 to n = 1
done
clear
C)
n = 3 to n = 2
done
clear
D)
n = 4 to n = 3
done
clear
View Answer play_arrow
question_answer 26) lonization energy of He+ ion at minimum position is
A)
13.6 eV
done
clear
B)
27.2 eV
done
clear
C)
54.4 eV
done
clear
D)
68.0 eV
done
clear
View Answer play_arrow
question_answer 27) The half-life period of a radioactive substance is 3 days. Three fourth of substance decays in
A)
3 days
done
clear
B)
6 days
done
clear
C)
9 days
done
clear
D)
12 days
done
clear
View Answer play_arrow
question_answer 28) If the decay constant of a radioactive substance is \[\lambda \], then its half-life is
A)
\[\frac{1}{\lambda }{{\log }_{e}}2\]
done
clear
B)
\[\frac{1}{\lambda }\]
done
clear
C)
\[\lambda {{\log }_{e}}2\]
done
clear
D)
\[\frac{\lambda }{{{\log }_{e}}2}\]
done
clear
View Answer play_arrow
question_answer 29) A galvanometer can be converted into a voltmeter by connecting
A)
low resistance in parallel
done
clear
B)
low resistance in series
done
clear
C)
high resistance in parallel
done
clear
D)
high resistance in series
done
clear
View Answer play_arrow
question_answer 30) A heater coil cut into two equal parts and one part is connected with heater. Now heat generated in heater will be
A)
twice
done
clear
B)
half
done
clear
C)
one- fourth
done
clear
D)
four times
done
clear
View Answer play_arrow
question_answer 31) A particle moves along with x-axis. The position\[x\]of particle with respect to time t from origin given by \[x={{b}_{0}}+{{b}_{1}}t+{{b}_{2}}{{t}_{2}}\]. The acceleration of particle is
A)
\[{{b}_{0}}\]
done
clear
B)
\[{{b}_{1}}\]
done
clear
C)
\[{{b}_{2}}\]
done
clear
D)
\[2{{b}_{2}}\]
done
clear
View Answer play_arrow
question_answer 32) One end of string of length\[l\]is connected to a particle of mass m and the other to a small peg on a smooth horizontal table. If the particle moves in a circle with speed v, the net force on the particle (directed towards the centre) is
A)
zero
done
clear
B)
\[T-\frac{m{{v}^{2}}}{I}\]
done
clear
C)
\[T\]
done
clear
D)
\[T+\frac{m{{v}^{2}}}{I}\]
done
clear
View Answer play_arrow
question_answer 33) If the kinetic energy of a body is increased 2 times, its momentum will
A)
half
done
clear
B)
remain unchanged
done
clear
C)
be doubled
done
clear
D)
increase \[\sqrt{2}\]times
done
clear
View Answer play_arrow
question_answer 34) The moment of inertia of a solid sphere of mass M and radius R about the tangent on its surface is
A)
\[\frac{7}{5}M{{R}^{2}}\]
done
clear
B)
\[\frac{4}{5}M{{R}^{2}}\]
done
clear
C)
\[\frac{2}{5}M{{R}^{2}}\]
done
clear
D)
\[\frac{1}{2}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 35) Satellites A and B are revolving around the orbit of earth. The mass of A is 10 times to mass of B. The ratio of time period \[\left( \frac{{{T}_{A}}}{{{T}_{B}}} \right)\] is
A)
\[10\]
done
clear
B)
\[1\]
done
clear
C)
\[\frac{1}{5}\]
done
clear
D)
\[\frac{1}{10}\]
done
clear
View Answer play_arrow
question_answer 36) A particle of amplitude A is executing simple harmonic motion. When the potential energy of particle is half of its maximum potential energy, then displacement from its equilibrium position is
A)
\[\frac{A}{4}\]
done
clear
B)
\[\frac{A}{3}\]
done
clear
C)
\[\frac{A}{2}\]
done
clear
D)
\[\frac{A}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 37) A simple wave motion represents by\[y=5(\sin 4\pi t+\sqrt{3}\cos 4\pi t)\]. Its amplitude is
A)
5
done
clear
B)
5\[\sqrt{3}\]
done
clear
C)
\[10\sqrt{3}\]
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 38) A liquid does not wet the sides of a solid, if the angle of contact is
A)
obtuse
done
clear
B)
\[90{}^\circ \]
done
clear
C)
acute
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 39) Root means square speed of the molecules of ideal gas is\[v\]. If pressure is increased two times at constant temperature, then the rms speed will become
A)
\[\frac{v}{2}\]
done
clear
B)
\[v\]
done
clear
C)
\[2v\]
done
clear
D)
\[4v\]
done
clear
View Answer play_arrow
question_answer 40) 1 mole of gas occupies a volume of 200 ml at 100 mm pressure. What is the volume occupied by two moles of gas at 400 mm pressure and at same temperature?
A)
50 mL
done
clear
B)
100 mL
done
clear
C)
200 mL
done
clear
D)
400 mL
done
clear
View Answer play_arrow
question_answer 41) The\[s{{p}^{3}}\]hybridisation is present on the central atom of
A)
\[HCHO\]
done
clear
B)
\[BC{{l}_{3}}\]
done
clear
C)
\[PC{{l}_{3}}\]
done
clear
D)
\[S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 42) The number of unit cells in the 5.85 g crystals of\[NaCl\]are
A)
\[1.5\times {{10}^{23}}\]
done
clear
B)
\[1.5\times {{10}^{22}}\]
done
clear
C)
\[3.0\times {{10}^{22}}\]
done
clear
D)
\[3.0\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 43) The oxidation number of sulphur is -1 in
A)
\[{{H}_{2}}S\]
done
clear
B)
\[Fe{{S}_{2}}\]
done
clear
C)
\[C{{S}_{2}}\]
done
clear
D)
\[C{{u}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 44) For which of the following metals, the electronic configuration of valence shell is not \[{{d}^{6}}\]?
A)
Fe (II)
done
clear
B)
\[Mn\](II)
done
clear
C)
Co (III)
done
clear
D)
Ni (IV)
done
clear
View Answer play_arrow
question_answer 45) 68 g sugar\[({{C}_{12}}{{H}_{22}}{{O}_{11}})\]is dissolved in 1 kg of water. What is the mole fraction of sugar?
A)
0.018
done
clear
B)
0.036
done
clear
C)
0.0018
done
clear
D)
0.0036
done
clear
View Answer play_arrow
question_answer 46) The pH of 0.1 M aqueous ammonia \[({{K}_{b}}=1.8\times {{10}^{-5}})\]is.
A)
9.13
done
clear
B)
10.13
done
clear
C)
11.13
done
clear
D)
12.13
done
clear
View Answer play_arrow
question_answer 47) From the following mixtures, which is not a buffer (concentration level 0.5 M)?
A)
\[C{{H}_{3}}COOH+NaOH(2:1)\]
done
clear
B)
\[HCl+N{{H}_{3}}(aq)(1:2)\]
done
clear
C)
\[C{{H}_{3}}COOH+NaOH(1:2)\]
done
clear
D)
\[HCl+N{{H}_{3}}(2:3)\]
done
clear
View Answer play_arrow
question_answer 48) How much volume (in litre) of\[3M\,NaOH\]is obtained from 80 g\[NaOH\]? (Atomic mass of \[Na=23u\])
A)
2.67
done
clear
B)
1.34
done
clear
C)
0.67
done
clear
D)
0.33
done
clear
View Answer play_arrow
question_answer 49) The initial concentration of sugar solution is 0.12 M. On doing fermentation the concentration of sugar decreases to 0.06 M in 10 h and to 0.045 M in 15 h. The order of the reaction is
A)
0.5
done
clear
B)
1.0
done
clear
C)
1.5
done
clear
D)
2.0
done
clear
View Answer play_arrow
question_answer 50) For the decomposition of\[{{N}_{2}}O\]and\[{{O}_{2}}\]in presence of at, the velocity constant, k is \[k=5\times {{10}^{11}}{{e}^{-30,000/T}}\] For this, the activation energy is (in kJ\[mo{{l}^{-1}}\])
A)
2.494
done
clear
B)
24.94
done
clear
C)
249.4
done
clear
D)
2494
done
clear
View Answer play_arrow
question_answer 51) The following equilibrium establishes on heating 0.2 mole of\[{{H}_{2}}\]and 1.0 mole of sulphur in 1 L vessel at\[90{}^\circ C\]. \[{{H}_{2}}(g)+S(s){{H}_{2}}S(g);\] \[K=6.8\times {{10}^{-2}}\] The partial pressure of\[{{H}_{2}}S\]in equilibrium state is
A)
4.20
done
clear
B)
0.42
done
clear
C)
0.21
done
clear
D)
0.042
done
clear
View Answer play_arrow
question_answer 52) An aqueous solution boils at\[100.2{}^\circ C\]. At which temperature this will freeze. \[({{K}_{b}}=0.5{}^\circ C/m,{{K}_{f}}=1.9{}^\circ C/m)\]
A)
\[+0.76\]
done
clear
B)
\[-0.76\]
done
clear
C)
\[-0.38\]
done
clear
D)
\[+0.38\]
done
clear
View Answer play_arrow
question_answer 53) The lowest\[p{{K}_{a}}\]value is for
A)
phenol
done
clear
B)
\[m-\]cresol
done
clear
C)
o-cresol
done
clear
D)
p-cresol
done
clear
View Answer play_arrow
question_answer 54) At room temperature, the least stable compound is
A)
\[C{{H}_{3}}COCl\]
done
clear
B)
\[HCOCl\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 55) For the conversion of\[C{{H}_{2}}=C{{H}_{2}}\]into\[HOOC\]. \[C{{H}_{2}}C{{H}_{2}}COOH,\]the minimum number of steps required are
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 56) Which of the following compounds does not give two isomer compounds on reaction with\[N{{H}_{2}}OH\]?
A)
\[C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[PhCOC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 57) From the following which is not a reactant, reagent or product in Hoffmann reaction?
A)
\[RCON{{H}_{2}}\]
done
clear
B)
\[RN{{H}_{2}}\]
done
clear
C)
\[B{{r}_{2}},O{{H}^{-}}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 58) The total number of isomers for cyclic alcohol \[{{C}_{4}}{{H}_{7}}OH\]is
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 59) The least stable free radical is
A)
\[\overset{\bullet }{\mathop{C}}\,{{H}_{2}}C{{H}_{2}}CH{{(C{{H}_{3}})}_{2}}\]
done
clear
B)
\[C{{H}_{3}}\underset{\bullet }{\mathop{C}}\,HCH{{(C{{H}_{3}})}_{2}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}\underset{\bullet }{\mathop{C}}\,{{(C{{H}_{3}})}_{2}}\]
done
clear
D)
\[\overset{\bullet }{\mathop{C}}\,{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 60) The suitable reagent for the reduction of \[{{C}_{2}}{{H}_{5}}COOH\]into\[{{C}_{3}}{{H}_{7}}OH\]is
A)
\[B{{H}_{3}}/THF\]and\[{{H}_{3}}{{O}^{+}}\]
done
clear
B)
\[NaB{{H}_{4}}\]
done
clear
C)
\[Na/EtOH\]
done
clear
D)
\[{{H}_{2}}/\]catalyst
done
clear
View Answer play_arrow
question_answer 61) The blue colour of acidic solution of\[C{{r}_{2}}O_{7}^{2-}\]is not changed into green by
A)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
View Answer play_arrow
question_answer 62) The reaction of\[{{C}_{2}}{{H}_{5}}Cl\]with\[Li\]and\[CuI\]gives mainly
A)
2-butene
done
clear
B)
1, 3-butadiene
done
clear
C)
n-butane
done
clear
D)
n-butyl chloride
done
clear
View Answer play_arrow
question_answer 63) The reagent which does not convert n-butyl chloride into zi-butane is
A)
\[Zn,\text{ }HCl\]
done
clear
B)
\[LIAl{{H}_{4}}\]
done
clear
C)
\[Mg,\]Anhydrous ether,\[{{H}_{2}}O\]
done
clear
D)
\[{{B}_{2}}{{H}_{6}}\]in THF
done
clear
View Answer play_arrow
question_answer 64) The correct order of stability is
A)
Pentane < iso-pentane < neo-pentane
done
clear
B)
iso-pentane < neo-pentane < pentane
done
clear
C)
neo-pentane < iso-pentane < pentane
done
clear
D)
Pentane < iso-pentane < iso-pentane
done
clear
View Answer play_arrow
question_answer 65) The correct order of boiling point of ethyl dimethyl amine , n-butyl amine and diethyl amine is
A)
\[B>C>A\]
done
clear
B)
\[B>A>C\]
done
clear
C)
\[A>B>C\]
done
clear
D)
\[C>B>A\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following compounds does not give iodoform test?
A)
\[C{{H}_{3}}COC{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[PhC{{H}_{2}}COC{{H}_{3}}\]
done
clear
C)
\[M{{e}_{3}}C.COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 67) The species which acts as both nucleophile and electrophile is
A)
\[C{{H}_{3}}CN\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[P{{(C{{H}_{3}})}_{2}}\]
done
clear
D)
\[{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 68) The most basic from the following is
A)
\[N{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[N{{F}_{3}}\]
done
clear
D)
\[N{{(Si{{H}_{3}})}_{3}}\]
done
clear
View Answer play_arrow
question_answer 69) The main product of the reaction of benzene with lithium in liquid ammonia and\[EtOH\]is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 70) The rate of free radical chlorination of\[C{{H}_{4}}\]is
A)
equal to\[C{{D}_{4}}\]
done
clear
B)
double the rate of \[C{{D}_{4}}\]
done
clear
C)
12 times the rate of\[C{{D}_{4}}\]
done
clear
D)
less than the rate of\[C{{D}_{4}}\]
done
clear
View Answer play_arrow
question_answer 71) The first ionisation energy difference is maximum for which pair?
A)
\[Na,Mg\]
done
clear
B)
\[K,Ca\]
done
clear
C)
\[Rb,Sr\]
done
clear
D)
\[Cs,Ba\]
done
clear
View Answer play_arrow
question_answer 72) The ratio of third Bohr orbit radius and second Bohr orbit radius for the hydrogen atom is
A)
0.5
done
clear
B)
1.5
done
clear
C)
0.75
done
clear
D)
2.25
done
clear
View Answer play_arrow
question_answer 73) The principal quantum number of Mn for those valence shell orbits in which electrons are filled
A)
4, 3
done
clear
B)
4, 4
done
clear
C)
3, 3
done
clear
D)
5, 4
done
clear
View Answer play_arrow
question_answer 74) From the generally known oxidation states, the oxidation number is maximum for
A)
\[Mn\]
done
clear
B)
\[Cu\]
done
clear
C)
\[Sn\]
done
clear
D)
\[Sc\]
done
clear
View Answer play_arrow
question_answer 75) The number of molecules in 180 g of heavy water are
A)
\[6.02\times {{10}^{24}}\]
done
clear
B)
\[6.02\times {{10}^{22}}\]
done
clear
C)
\[5.42\times {{10}^{24}}\]
done
clear
D)
\[5.42\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following is reduced by\[{{H}_{2}}{{O}_{2}}\]?
A)
\[C{{l}_{2}}\]
done
clear
B)
\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
done
clear
C)
\[N{{H}_{2}}OH\]
done
clear
D)
\[SO_{3}^{2-}\]
done
clear
View Answer play_arrow
question_answer 77) The maximum energy molecular orbit filled by electron in nitrogen molecule is/are
A)
\[\sigma 2{{p}_{z}}\]
done
clear
B)
\[\pi 2{{p}_{x}}\approx \pi 2{{p}_{y}}\]
done
clear
C)
\[{{\pi }^{*}}2{{p}_{x}}\approx {{\pi }^{*}}2{{p}_{y}}\]
done
clear
D)
\[{{\sigma }^{*}}2{{p}_{z}}\]
done
clear
View Answer play_arrow
question_answer 78) Which of the following is correct order for density?
A)
\[Cs>Rb>K>Na\]
done
clear
B)
\[Cs>Rb>Na>K\]
done
clear
C)
\[Rb>Cs>K>Na\]
done
clear
D)
\[Rb>Cs>Na>K\]
done
clear
View Answer play_arrow
question_answer 79) The correct order of dipole moment is
A)
\[B{{F}_{3}}<{{H}_{2}}S<{{H}_{2}}O\]
done
clear
B)
\[{{H}_{2}}S<B{{F}_{3}}<{{H}_{2}}O\]
done
clear
C)
\[{{H}_{2}}O<{{H}_{2}}S<B{{F}_{3}}\]
done
clear
D)
\[{{H}_{2}}O<B{{F}_{3}}<{{H}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 80) Which of the following bond has minimum bond energy?
A)
\[C-H\]
done
clear
B)
\[N-H\]
done
clear
C)
\[O-H\]
done
clear
D)
\[F-H\]
done
clear
View Answer play_arrow
question_answer 81) The intersection point of the normals drawn at the end points of latusrectum of the parabola\[{{x}^{2}}=-2y\]is
A)
\[\left( -\frac{1}{2},-\frac{3}{2} \right)\]
done
clear
B)
\[\left( \frac{1}{2},-\frac{3}{2} \right)\]
done
clear
C)
\[(0,-1)\]
done
clear
D)
\[\left( 0,-\frac{3}{2} \right)\]
done
clear
View Answer play_arrow
question_answer 82) The equation whose roots are reciprocal of the roots of the equation\[a{{x}^{2}}+bx+c=0,\]is
A)
\[b{{x}^{2}}+cx+a=0\]
done
clear
B)
\[b{{x}^{2}}+ax+c=0\]
done
clear
C)
\[c{{x}^{2}}+ax+b=0\]
done
clear
D)
\[c{{x}^{2}}+bx+a=0\]
done
clear
View Answer play_arrow
question_answer 83) If in the expansion of\[{{\left( 3x-\frac{2}{{{x}^{2}}} \right)}^{15}},\]rth term is independent of\[x,\]then value of r is
A)
11
done
clear
B)
10
done
clear
C)
9
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 84) If\[f(x)=\left| \begin{matrix} 1+a & 1-ax & 1+a{{x}^{2}} \\ 1+b & 1+bx & 1+b{{x}^{2}} \\ 1+c & 1+cx & 1+c{{x}^{2}} \\ \end{matrix} \right|,\]where\[a,b,c\] are non-zero Constants, then value of\[f(10)\] is
A)
\[10(b-a)(c-a)\]
done
clear
B)
\[100(b-a)(c-b)(a-c)\]
done
clear
C)
\[100\,abc\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 85) If\[^{n}{{C}_{12}}{{=}^{n}}{{C}_{8}},\]then value of\[^{n}{{C}_{19}}\]is
A)
1
done
clear
B)
20
done
clear
C)
210
done
clear
D)
1540
done
clear
View Answer play_arrow
question_answer 86) If\[\omega \]is a complex cube root of unity and\[A=\left[ \begin{matrix} \omega & 0 \\ 0 & \omega \\ \end{matrix} \right],\]then\[{{A}^{50}}\]is
A)
\[{{\omega }^{2}}A\]
done
clear
B)
\[\omega A\]
done
clear
C)
\[A\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 87) If sum of\[n\]terms of an AP is\[2n+3{{n}^{2}},\]then rth term is
A)
\[2r+3{{r}^{2}}\]
done
clear
B)
\[3{{r}^{2}}-4r+1\]
done
clear
C)
\[6r-1\]
done
clear
D)
\[4r+1\]
done
clear
View Answer play_arrow
question_answer 88) If A and B are square matrices of order\[3\times 3,\] then which of the following is true?
A)
\[AB=0\Rightarrow A=O\]or\[B=O\]
done
clear
B)
\[det(2AB)=8\text{ (}detA)\text{ (}detB)\]
done
clear
C)
\[{{A}^{2}}-{{E}^{2}}=(A+B)(A-B)\]
done
clear
D)
\[det(A+B)=det(A)+det(B)\]
done
clear
View Answer play_arrow
question_answer 89) If geometric mean and harmonic mean between two different numbers are 12 and \[\frac{48}{5}\]respectively, then one number is
A)
4
done
clear
B)
6
done
clear
C)
8
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 90) Let a, b, c are in GP and 4a,5b,4c are in AP such that\[a+b+c=70,\]then value of\[b\]is
A)
5
done
clear
B)
10
done
clear
C)
15
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 91) The value of integral\[\int_{0}^{4}{|x-1|}dx\] is
A)
4
done
clear
B)
5
done
clear
C)
7
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 92) If\[\frac{x-1}{1+i}+\frac{y-1}{1-i}=i,\]where\[x\]and y are real numbers and\[i=\sqrt{-1},\]then
A)
\[x=0\]
done
clear
B)
\[x<0\]
done
clear
C)
\[x>0\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 93) If a pair of two fair dice is thrown, then the probability of getting the sum 5 on both dice is
A)
\[\frac{5}{36}\]
done
clear
B)
\[\frac{1}{12}\]
done
clear
C)
\[\frac{1}{18}\]
done
clear
D)
\[\frac{1}{9}\]
done
clear
View Answer play_arrow
question_answer 94) If a fair coin is tossed 20 times and let we get head n times, then probability that 21 is odd, is
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{6}\]
done
clear
C)
\[\frac{5}{8}\]
done
clear
D)
\[\frac{7}{8}\]
done
clear
View Answer play_arrow
question_answer 95) If\[{{x}_{r}}=\cos \left( \frac{\pi }{{{2}^{r}}} \right)+i\sin \left( \frac{\pi }{{{2}^{r}}} \right),\]then value of\[{{x}_{1}}\,{{x}_{2}}\,\,{{x}_{3}}....\]is
A)
\[i\]
done
clear
B)
\[1\]
done
clear
C)
\[-1\]
done
clear
D)
\[-i\]
done
clear
View Answer play_arrow
question_answer 96) If\[{{\left( \frac{1+\cos \phi +i\sin \phi }{1+\cos \phi -i\sin \phi } \right)}^{n}}=u+iv,\]where u and v are real numbers, then u is
A)
\[n\cos \phi \]
done
clear
B)
\[\cos n\phi \]
done
clear
C)
\[\cos \left( \frac{n\phi }{2} \right)\]
done
clear
D)
\[\sin \left( \frac{n\phi }{2} \right)\]
done
clear
View Answer play_arrow
question_answer 97) If square root of \[-7+24i\] is \[x+iy,\] then \[x\] is
A)
±1
done
clear
B)
± 2
done
clear
C)
±3
done
clear
D)
± 4
done
clear
View Answer play_arrow
question_answer 98) If \[{{\tanh }^{-1}}(x-iy)=\frac{1}{2}{{\tanh }^{-1}}\left( \frac{2x}{1+{{x}^{2}}+{{y}^{2}}} \right)\] \[+\frac{i}{2}{{\tan }^{-1}}\left( \frac{2y}{1-{{x}^{2}}-{{y}^{2}}} \right)x,y\in R,\] then \[{{\tanh }^{-1}}(iy)\] is
A)
\[2\text{ }tan{{h}^{-1}}(y)\]
done
clear
B)
\[-2\text{ }tan{{h}^{-1}}(y)\]
done
clear
C)
\[\text{i }ta{{n}^{-1}}y\]
done
clear
D)
\[\text{-i }ta{{n}^{-1}}(y)\]
done
clear
View Answer play_arrow
question_answer 99) If \[f:R\to R\] is defined as \[f(x)={{(1-x)}^{1/3}}\] then \[{{f}^{-1}}(x)\] is
A)
\[{{(1-x)}^{-1/3}}\]
done
clear
B)
\[{{(1-x)}^{3}}\]
done
clear
C)
\[1-{{x}^{3}}\]
done
clear
D)
\[1-{{x}^{1/3}}\]
done
clear
View Answer play_arrow
question_answer 100) If \[{{x}^{y}}={{e}^{x-y}},x>0,\] then value of \[\frac{dy}{dx}\] at (1,1) is
A)
0
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 101) The value of \[\underset{x\to 0}{\mathop{\lim }}\,\frac{(1-\cos 2x)}{{{x}^{2}}}\]is
A)
doesn't exist
done
clear
B)
infinite
done
clear
C)
0
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 102) The value of differentiation of\[{{e}^{{{x}^{2}}}}\]w.r.t. to \[{{e}^{2x-1}}\]at\[x=1\]is
A)
e
done
clear
B)
0
done
clear
C)
\[{{e}^{-1}}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 103) If function\[f(x),x\in R\]is differentiable and \[f(1)=1,\]then value of\[\underset{x\to 0}{\mathop{\lim }}\,\frac{(1-\cos 2x)}{{{x}^{2}}}\]is
A)
0
done
clear
B)
1
done
clear
C)
\[f'(1)\]
done
clear
D)
\[\infty \]
done
clear
View Answer play_arrow
question_answer 104) The value of integral \[\int_{0}^{2a}{\frac{f(x)}{f(x)+f(2a-x)}}dx,\]where\[f(x)\]is a continuous function, is
A)
0
done
clear
B)
1
done
clear
C)
\[a\]
done
clear
D)
\[2a\]
done
clear
View Answer play_arrow
question_answer 105) The area of the region bounded by the curves \[y=ex,\text{ y}=lo{{g}_{e}}x\]and lines\[x=1,\text{ }x=2\]is
A)
\[{{(e-1)}^{2}}\]
done
clear
B)
\[{{e}^{2}}-e+1\]
done
clear
C)
\[{{e}^{2}}-e+1-2lo{{g}_{e}}2\]
done
clear
D)
\[{{e}^{2}}+e-2lo{{g}_{e}}2\]
done
clear
View Answer play_arrow
question_answer 106) If\[f(x)=sin\text{ }x+cos\text{ }x+1\]and \[g(x)={{x}^{2}}+x,\text{ }x\in R,\]then value of\[fog(x)\]at \[x=0\]is
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 107) The maximum value of function \[f(x)=\sin x(1+\cos x),x\in R\]is
A)
\[\frac{{{3}^{3/2}}}{4}\]
done
clear
B)
\[\frac{{{3}^{5/3}}}{4}\]
done
clear
C)
\[\frac{3}{2}\]
done
clear
D)
\[\frac{{{3}^{7/5}}}{4}\]
done
clear
View Answer play_arrow
question_answer 108) The normal at point (1,1) to the curve\[{{y}^{2}}={{x}^{3}}\]is parallel to the line
A)
\[3x-y-2=0\]
done
clear
B)
\[2x+3y-7=0\]
done
clear
C)
\[2x-3y+1=0\]
done
clear
D)
\[2y-3x+1=0\]
done
clear
View Answer play_arrow
question_answer 109) Function\[f(x)=|x-1|+|x-2|,x\in R\]is
A)
differentiable everywhere in R
done
clear
B)
except\[x=1\]and\[x=2\]differentiable everywhere in R
done
clear
C)
not continuous at\[x=1\]and\[x=2\]
done
clear
D)
increasing in\[R\]
done
clear
View Answer play_arrow
question_answer 110) If\[f(1)=2\]and\[f'(1)=1,\]then the value of\[\underset{x\to 1}{\mathop{\lim }}\,\frac{2x-f(x)}{x-1}\]is
A)
\[-1\]
done
clear
B)
0
done
clear
C)
\[1\]
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 111) The distance of the point P (1,2,3) from the line which passes through the point A (4,2,2) and parallel to the vector\[2\hat{i}+3\hat{j}+6\hat{k},\]is
A)
\[\sqrt{10}\]
done
clear
B)
\[\sqrt{7}\]
done
clear
C)
\[\sqrt{5}\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 112) Let\[\overrightarrow{a}=2\hat{i}+\hat{k},\overrightarrow{b}=\hat{i}+\hat{i}+\hat{k}\]and\[\overrightarrow{c}=4\hat{i}-3\hat{j}+7\hat{k}\]. If r is a vector such that\[\overrightarrow{r}\times \overrightarrow{b}=\overrightarrow{c}\times \overrightarrow{b}\] and\[\overrightarrow{r}.\overrightarrow{a}=0,\]then value of\[\overrightarrow{r}.\overrightarrow{b}\]is
A)
7
done
clear
B)
\[-7\]
done
clear
C)
\[-5\]
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 113) A unit vector which is coplanar with \[\hat{i}+\hat{j}+2\hat{k}\]and\[\hat{i}+2\hat{j}+\hat{k}\]and perpendicular to \[\hat{i}+\hat{j}+\hat{k},\] is
A)
\[\frac{(-\hat{j}+\hat{k})}{\sqrt{2}}\]
done
clear
B)
\[\frac{(\hat{k}-\hat{i})}{\sqrt{2}}\]
done
clear
C)
\[\frac{(\hat{i}-\hat{j})}{\sqrt{2}}\]
done
clear
D)
\[\frac{(\hat{i}-\hat{k})}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 114) If a line is inclined at\[45{}^\circ \]to both x-axis and\[y-\]axis, then the angle at which it is inclined to z-axis is
A)
\[45{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[30{}^\circ \]
done
clear
D)
\[90{}^\circ \]
done
clear
View Answer play_arrow
question_answer 115) The equation of plane which passes through the point\[(1,2,-1)\]and parallel to the plane \[x-y+2z=0\]is
A)
\[x-y+2z-3=0\]
done
clear
B)
\[x-y+2z+3=0\]
done
clear
C)
\[x-y+2z+6=0\]
done
clear
D)
\[x-y+2z-7=0\]
done
clear
View Answer play_arrow
question_answer 116) Let a plane passes through the point P(-1, -1,1) and also passes through a line joining the points Q (0,1,1) and R(Q,0,2). Then, the distance of plane from the point (0,0,0) is
A)
3
done
clear
B)
0
done
clear
C)
\[1/\sqrt{6}\]
done
clear
D)
\[2/\sqrt{6}\]
done
clear
View Answer play_arrow
question_answer 117) If\[f(x)\]and\[g(x),x\in R\]are continuous functions, then value of integral \[\int_{-\pi /2}^{\pi /2}{[\{f(x)+f(-x)\}\{g(x)-g(-x)\}]}\,dx\]is
A)
\[\pi \]
done
clear
B)
\[\pi /2\]
done
clear
C)
\[1\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 118) The equation of circle which touches the\[x-\]axis and 7-axis at points (1, 0) and (0, 1) respectively, is
A)
\[{{x}^{2}}+{{y}^{2}}-4y+3=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}-2y-2=0\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}-2x-27+2=0\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}-2x-2y+1=0\]
done
clear
View Answer play_arrow
question_answer 119) If lines\[4x+3y=1,\text{ }y-x=5\]and\[kx+5y=1\]are concurrent, then value of k is
A)
0
done
clear
B)
1
done
clear
C)
3
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 120) The equation\[{{y}^{2}}-8y-x+19=0\]represents
A)
a parabola whose focus is\[\left( \frac{1}{4},0 \right)\]and directrix is\[x=-\frac{1}{4}\]
done
clear
B)
a parabola whose vertex is (3, 4) and directrix is\[x=\frac{11}{4}\]
done
clear
C)
a parabola whose focus is\[\left( \frac{13}{4},4 \right)\]and vertex is (0, 0)
done
clear
D)
a curve which is not a parabola
done
clear
View Answer play_arrow