A) weak alkaline
B) acidic
C) strong alkaline
D) neutral
Correct Answer: C
Solution :
\[C{{H}_{3}}COONaC{{H}_{3}}CO{{O}^{-}}+N{{a}^{+}}\]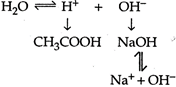 Due to this reason \[C{{H}_{3}}COONa\]solution will be basic so strong alkaline.
Due to this reason \[C{{H}_{3}}COONa\]solution will be basic so strong alkaline.
You need to login to perform this action.
You will be redirected in
3 sec
