A) \[CC{{l}_{4}}\]
B) \[PC{{l}_{5}}\]
C) \[B{{F}_{3}}\]
D) \[S{{F}_{6}}\]
Correct Answer: C
Solution :
The compounds in which central atom has less than 8 electrons in their valence shell are electron deficient compounds. (a)\[CC{{l}_{4}}Cl-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-Cl\] Carbon (central atom) has 8 electrons in valence shell, 4 from carbon and 1 electron each from four chlorine atom. (b)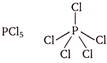 Phosphorus (central atom) has 10 electrons in valence shell, 5 from P and one each from five chlorine atoms. (c)
Phosphorus (central atom) has 10 electrons in valence shell, 5 from P and one each from five chlorine atoms. (c) 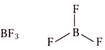 Boron (central atom) has 6 electrons in valence shell, 3 from boron and one each from three fluorine atoms. \[\because \]Boron in \[B{{F}_{3}}\] has less than 8 electrons. \[\therefore \]It is electron deficient molecule and is correct answer. (d)
Boron (central atom) has 6 electrons in valence shell, 3 from boron and one each from three fluorine atoms. \[\because \]Boron in \[B{{F}_{3}}\] has less than 8 electrons. \[\therefore \]It is electron deficient molecule and is correct answer. (d) 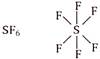 Sulphur (central atom) has 12 electrons in valence shell, 6 from sulphur and one each from six fluorine atoms.
Sulphur (central atom) has 12 electrons in valence shell, 6 from sulphur and one each from six fluorine atoms.
You need to login to perform this action.
You will be redirected in
3 sec
