A) \[C{{H}_{3}}-C{{H}_{2}}-Cl+O{{H}^{-}}\]
B) \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,l-C{{H}_{3}}+O{{H}^{-}}\]
C) \[C{{H}_{3}}-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}+O{{H}^{-}}\]
D) \[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}+O{{H}^{-}}\]
Correct Answer: A
Solution :
\[{{S}_{N}}2\]mechanism means bimolecular nucleophilic substitution mechanism. In this, rate of reaction is determined by concentration of two molecules. It is characteristic of \[{{1}^{o}}\]alkyl halides. (a)\[\underset{{{1}^{o}}alkyl\,\,halide}{\mathop{C{{H}_{3}}-C{{H}_{2}}-Cl+O{{H}^{-}}}}\,\] (b)\[\underset{{{3}^{o}}alkyl\,\,halide}{\mathop{C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}+OH}}\,\] (c)\[\underset{{{2}^{o}}alkyl\,\,halide}{\mathop{C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}+O{{H}^{-}}}}\,\] (d)\[\underset{{{3}^{o}}\,\,alkyl\,\,halide}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}+O{{H}^{-}}}}\,\]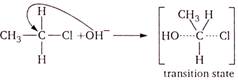 \[OH-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} \downarrow \\ C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-H+C{{l}^{-}}\]
\[OH-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} \downarrow \\ C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-H+C{{l}^{-}}\]
You need to login to perform this action.
You will be redirected in
3 sec
