A) \[V\]
B) \[Cr\]
C) \[Mn\]
D) \[Fe\]
Correct Answer: B
Solution :
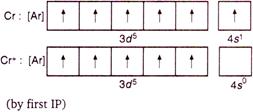 This is stable\[EC\], hence formation of \[C{{r}^{2+}}\] by second \[IP\] requires maximum enthalpy.
This is stable\[EC\], hence formation of \[C{{r}^{2+}}\] by second \[IP\] requires maximum enthalpy.
You need to login to perform this action.
You will be redirected in
3 sec
