A) \[{{B}_{2}}{{H}_{6}}\]
B) \[N{{H}_{3}}\]
C) \[{{C}_{2}}{{H}_{6}}\]
D) \[CC{{l}_{4}}\]
Correct Answer: A
Solution :
\[{{B}_{2}}{{H}_{6}}\] is an electron deficient compound in which \[B\] is \[s{{p}^{3}}\] hybridized state. It has the following \[H-\]bridged structure.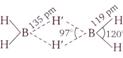
You need to login to perform this action.
You will be redirected in
3 sec
