| (i) \[Xe{{O}_{3}}\] |
| (ii)\[Xe{{O}_{4}}\] |
| (iii)\[Xe{{F}_{6}}\] |
A) (i) and (iii) only
B) (i) and (ii) only
C) (ii) and (iii) only
D) (i), (ii) and (iii)
Correct Answer: B
Solution :
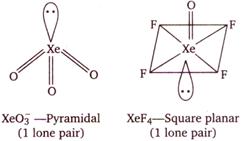
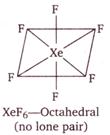 Hence, \[Xe{{O}_{3}}\] and \[Xe{{O}_{4}}\] have same number of lone pair\[(1\,\,\text{lp})\]on\[Xe\].
Hence, \[Xe{{O}_{3}}\] and \[Xe{{O}_{4}}\] have same number of lone pair\[(1\,\,\text{lp})\]on\[Xe\].
You need to login to perform this action.
You will be redirected in
3 sec
