A) methyl group
B) carboxylic acid group
C) methylene group
D) bicarbonate
Correct Answer: D
Solution :
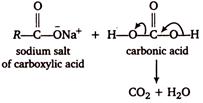
During the reaction of carboxylic acids with weaker base \[NaHC{{O}_{3}}\], the \[C{{O}_{2}}\] evolved comes from \[NaHC{{O}_{3}}\] and not from the carboxylic acid as shown below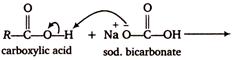
You need to login to perform this action.
You will be redirected in
3 sec
