question_answer 1) Dimension of relative density is:
A)
\[[M{{L}^{2}}]\]
done
clear
B)
\[[M{{L}^{-3}}]\]
done
clear
C)
dimension less
done
clear
D)
\[[{{M}^{2}}{{L}^{-6}}]\]
done
clear
View Answer play_arrow
question_answer 2) A ball is thrown from height h and another from \[2h\] The ratio of time taken by the two balls to reach ground is:
A)
\[1:\sqrt{2}\]
done
clear
B)
\[\sqrt{2}:1\]
done
clear
C)
2 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 3) A ball is thrown upwards, it takes 4 s to reach back to the ground. Find its initial velocity :
A)
\[30\,m{{s}^{-1}}\]
done
clear
B)
\[10\,m{{s}^{-1}}\]
done
clear
C)
\[40\,m{{s}^{-1}}\]
done
clear
D)
\[20\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 4) A man is at a height of 100 m. He sees a car which makes an angle of \[\frac{\pi }{6}\]with mans position. If the car moves to a point where angle is\[\frac{\pi }{3,}\] what is the distance moved by it?
A)
\[\left( \frac{100}{\sqrt{3}} \right)m\]
done
clear
B)
\[(200\sqrt{3})m\]
done
clear
C)
\[\left( \frac{200}{\sqrt{3}} \right)m\]
done
clear
D)
\[\left( \frac{150}{\sqrt{3}} \right)m\]
done
clear
View Answer play_arrow
question_answer 5) A body of mass 0.1 kg attains a velocity of \[10\,M{{s}^{-1}}\] in 0.1 s. The force acting on the body is :
A)
10 N
done
clear
B)
0.01 N
done
clear
C)
0.1 N
done
clear
D)
100 N
done
clear
View Answer play_arrow
question_answer 6) If force\[\mathbf{\vec{F}=5\vec{i}+3\vec{j}+4\vec{k}}\]makes a displacement of \[\mathbf{\vec{s}=}6\mathbf{\vec{i}}-5\mathbf{\vec{k}}\] work done by the force is :
A)
10 unit
done
clear
B)
\[122\sqrt{5}\] unit
done
clear
C)
\[5\sqrt{122}\] unit
done
clear
D)
20 unit
done
clear
View Answer play_arrow
question_answer 7) The angular velocity of second hand, of a clock is :
A)
\[\left( \frac{\pi }{6} \right)rad\,{{s}^{-1}}\]
done
clear
B)
\[\left( \frac{\pi }{60} \right)rad\,{{s}^{-1}}\]
done
clear
C)
\[\left( \frac{\pi }{30} \right)rad\,{{s}^{-1}}\]
done
clear
D)
\[\left( \frac{\pi }{15} \right)rad\,{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 8) A body is moving in a circular path with acceleration a. If its velocity gets doubled, find the ratio of acceleration after and before the change :
A)
1 : 4
done
clear
B)
\[\frac{1}{4}:1\]
done
clear
C)
2 : 1
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 9) Acceleration due to gravity at earths surface is \[g\,rn{{s}^{-2}}.\] Find the effective value of gravity at a height of 32 km from se. a level: \[({{R}_{e}}=6400\,km)\]
A)
\[0.5\,g\,m{{s}^{-2}}\]
done
clear
B)
\[0.99\,g\,m{{s}^{-2}}\]
done
clear
C)
\[1.01\,g\,m{{s}^{-2}}\]
done
clear
D)
\[0.90\,g\,m{{s}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 10) Near earths surface, time period of a stellite is 4 h. Find its time period at height 4R from the centre of earth :
A)
\[32\,h\]
done
clear
B)
\[\left( \frac{1}{8\sqrt[3]{2}} \right)h\]
done
clear
C)
\[8\sqrt[3]{2}\]
done
clear
D)
\[16\,h\]
done
clear
View Answer play_arrow
question_answer 11) Amplitude of a pendulum is 60 mm and angular velocity is 2 rad s-1. Find its velocity if its displacement is 22 mm:
A)
\[120\,\,mm\,{{s}^{-1}}\]
done
clear
B)
\[113\,\,mm\,{{s}^{-1}}\]
done
clear
C)
\[115\,\,mm\,{{s}^{-1}}\]
done
clear
D)
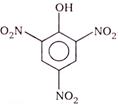
\[125\,\,mm\,{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 12) If we hollow the ball of pendulum, and fill it with sand, what would be the effect of change on the centre of mass ?
A)
it would distorted
done
clear
B)
its value will decrease only
done
clear
C)
its value will increase only
done
clear
D)
its value will first decrease and then increase
done
clear
View Answer play_arrow
question_answer 13) If n drops of potential V merge, find new potential on the big drop.
A)
\[{{n}^{2/3}}V\]
done
clear
B)
\[{{n}^{1/3}}V\]
done
clear
C)
\[nV\]
done
clear
D)
\[{{V}^{n/3}}\]
done
clear
View Answer play_arrow
question_answer 14) If relation between distance and time is \[s=a+bt+c{{t}^{2}},\]find initial velocity and acceleration :
A)
\[b+2ct,\,2c\]
done
clear
B)
\[b,\,\,2\,c\]
done
clear
C)
\[\,2\,c,\,b\]
done
clear
D)
\[\,b+2c,\,2c\]
done
clear
View Answer play_arrow
question_answer 15) If electric flux varies according to \[\phi =3{{t}^{2}}+4t+2,\] find emf at t = 2 s:
A)
22V
done
clear
B)
18 V
done
clear
C)
20 V
done
clear
D)
16 V
done
clear
View Answer play_arrow
question_answer 16) The means of energy transfer in vacuum is:
A)
irradiation
done
clear
B)
convection
done
clear
C)
radiation
done
clear
D)
conduction
done
clear
View Answer play_arrow
question_answer 17) One filament takes 10 min to heat a kettle and another takes 15 min. If connected in parallel they combindly take ............ min to heat the same kettle:
A)
6
done
clear
B)
12.5
done
clear
C)
25
done
clear
D)
7.5
done
clear
View Answer play_arrow
question_answer 18) Efficiency of engine working at \[40{}^\circ C\], \[20{}^\circ C\] is:
A)
0.064%
done
clear
B)
0.64%
done
clear
C)
6.4%
done
clear
D)
6.4%
done
clear
View Answer play_arrow
question_answer 19) If there is a straight line parallel to volume axis in a P-V diagram, then it is a .........graph.
A)
isochoric
done
clear
B)
isobaric
done
clear
C)
isothermal
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 20) In an adibatic process :
A)
\[{{P}^{\gamma }}V=\]constant
done
clear
B)
\[P{{V}^{\gamma -1}}=\]constant
done
clear
C)
\[PV=\]constant
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 21) An object is placed at a distance of 10 cm from a convex lens of power 5D. Find the position of the image :
A)
- 20 cm
done
clear
B)
30 cm
done
clear
C)
20 cm
done
clear
D)
- 30 cm
done
clear
View Answer play_arrow
question_answer 22) If separation between screen and source is in cresed by 2%, what would be the effect on the intensity?
A)
Increases by 4%
done
clear
B)
Increases by 2%
done
clear
C)
Decreases by 2%
done
clear
D)
Decreases by 4%
done
clear
View Answer play_arrow
question_answer 23) Magnification of a telescope having focal lengths of objective lens and eye piece\[{{f}_{0}}\]and\[{{f}_{e,}}\] respectively is:
A)
\[\frac{{{f}_{e}}}{{{f}_{0}}}\]
done
clear
B)
\[\frac{{{f}_{0}}}{{{f}_{e}}}\]
done
clear
C)
\[\frac{1-{{f}_{0}}}{{{f}_{e}}}\]
done
clear
D)
\[\frac{{{f}_{0}}}{{{f}_{e}}-1}\]
done
clear
View Answer play_arrow
question_answer 24) Maximum and minimum intensities obtained by. Two sources having intensities \[4I\] and \[I\]are:
A)
\[5I,-3I\]
done
clear
B)
\[9I,I\]
done
clear
C)
\[9I,-I\]
done
clear
D)
\[5I,3I\]
done
clear
View Answer play_arrow
question_answer 25) An equilateral prism has\[\mu =\sqrt{3.}\]Its angle of minimum deviation will be :
A)
\[30{}^\circ \]
done
clear
B)
\[{{60}^{\text{o}}}\]
done
clear
C)
\[{{120}^{\text{o}}}\]
done
clear
D)
\[{{45}^{\text{o}}}\]
done
clear
View Answer play_arrow
question_answer 26) . Separation between slits is halved and between secreen and slits is doubled. Final fringe width if original is w:
A)
w
done
clear
B)
9w
done
clear
C)
4w
done
clear
D)
2w
done
clear
View Answer play_arrow
question_answer 27) A source is approaching a stationary observer with velocity\[{{\left( \frac{1}{10} \right)}^{th}}\]that of sound. Ratio of observed and real frequencies will be :
A)
\[\frac{9}{10}\]
done
clear
B)
\[\frac{11}{10}\]
done
clear
C)
\[\frac{10}{11}\]
done
clear
D)
\[\frac{10}{9}\]
done
clear
View Answer play_arrow
question_answer 28) A source and observer are approaching each other with 50 ms-1 velocity. What will be original frequency if the observer receives 400 cycle/s?
A)
300 cycle/s
done
clear
B)
320 cycle/s
done
clear
C)
340 cycle/s
done
clear
D)
330 cycle/s
done
clear
View Answer play_arrow
question_answer 29) Distance between successive compression and rarefaction is 1 m and velocity of sound is 360 ms-1. Find frequency :
A)
180 Hz
done
clear
B)
45 Hz
done
clear
C)
120 Hz
done
clear
D)
90 Hz
done
clear
View Answer play_arrow
question_answer 30) A cube has point charges of magnitude -q at all its vertices. Electric field at the centre of the cube is:
A)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\frac{6q}{3{{a}^{2}}}\]
done
clear
B)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\frac{8q}{{{a}^{2}}}\]
done
clear
C)
zero
done
clear
D)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\frac{-8q}{{{a}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 31) Two conductors having current in opposite directions............
A)
attract each other
done
clear
B)
repel each other
done
clear
C)
do not affect each other
done
clear
D)
effect depends on the material of wire
done
clear
View Answer play_arrow
question_answer 32) If a capacitor of capacitance\[10\mu F\]has potential difference of 100 V across its ends, the energy stored in it is:
A)
0.05 J
done
clear
B)
1 J
done
clear
C)
0.005 J
done
clear
D)
0.1 J
done
clear
View Answer play_arrow
question_answer 33) Five resistances of resistance\[R\,\Omega \] are there, 3 are connected in parallel and are joined to them in series. Find resultant resistance:
A)
\[\left( \frac{3}{7} \right)R\,\Omega \]
done
clear
B)
\[\left( \frac{7}{3} \right)R\,\Omega \]
done
clear
C)
\[\left( \frac{7}{8} \right)R\,\Omega \]
done
clear
D)
\[\left( \frac{8}{7} \right)R\,\Omega \]
done
clear
View Answer play_arrow
question_answer 34) If reading of an ammeter is 10 A, the peak value of current is:
A)
\[\frac{10}{\sqrt{2}}A\]
done
clear
B)
\[\frac{5}{\sqrt{2}}A\]
done
clear
C)
\[20\sqrt{2}A\]
done
clear
D)
\[10\sqrt{2}A\]
done
clear
View Answer play_arrow
question_answer 35) If impedance is \[\sqrt{3}\] times of resistance, find phase difference :
A)
zero
done
clear
B)
\[30{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
data is incomplete
done
clear
View Answer play_arrow
question_answer 36) A bar magnet is dropped between a current carrying coil. What would be its acceleration?
A)
g downwards
done
clear
B)
greater than g downwards
done
clear
C)
less than g downwards
done
clear
D)
bar will be stationary
done
clear
View Answer play_arrow
question_answer 37) If the door of refrigerator is opened while connected to supply, the room gets :
A)
cooled
done
clear
B)
heated
done
clear
C)
no effect
done
clear
D)
temperature is not given
done
clear
View Answer play_arrow
question_answer 38) \[{{M}_{P}}\] and \[{{M}_{N}}\] are masses of proton and neutron, respectively, at rest. If they combine to form deuterium nucleus. The mass of the nucleus will be:
A)
less than \[{{M}_{P}}\]
done
clear
B)
less than \[({{M}_{P}}+{{M}_{N}})\]
done
clear
C)
less than \[({{M}_{P}}+2{{M}_{N}})\]
done
clear
D)
greater than \[({{M}_{P}}+2{{M}_{N}})\]
done
clear
View Answer play_arrow
question_answer 39) If resistance of wire at \[50{}^\circ C\] is \[5R\,\Omega \] and at \[100{}^\circ C\] is \[6R\,\Omega .\] find resistance of \[0{}^\circ C\] :
A)
\[0R\,\,\Omega \]
done
clear
B)
\[2R\,\,\Omega \]
done
clear
C)
\[3R\,\,\Omega \]
done
clear
D)
\[4R\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 40) The values \[+\frac{1}{2}\] and \[-\frac{1}{2}\] of spin quantum number show:
A)
rotation of \[{{e}^{-}}\] clockwise and anticlockwise direction respectively
done
clear
B)
rotation of \[{{e}^{-}}\] anticlockwise and clockwise directions respectively
done
clear
C)
rotation in any direction according to convention
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 41) If a sample of 16 g radioactive substance disintegrate to 1 g in 120 days, then what will be the half-life of the sample?
A)
15 days
done
clear
B)
7.5 days
done
clear
C)
30 days
done
clear
D)
60 days
done
clear
View Answer play_arrow
question_answer 42) If phosphorus and arsenic impurities are added to a semiconductor, then it becomes:
A)
transistor
done
clear
B)
p-type semiconductor
done
clear
C)
amplifier
done
clear
D)
n-type semiconductor
done
clear
View Answer play_arrow
question_answer 43) If velocity of a charged particle is doubled and strength of magnetic field is halved, then radius becomes :
A)
8 times
done
clear
B)
2 times
done
clear
C)
4 times
done
clear
D)
3 times
done
clear
View Answer play_arrow
question_answer 44) If kinetic energy is doubled, find fractional change in momentum :
A)
\[\sqrt{2}\]
done
clear
B)
\[2\sqrt{2}\]
done
clear
C)
\[\frac{1}{\sqrt{2}}\]
done
clear
D)
\[\frac{1}{2\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 45) At what temperature volume of an ideal gas at \[0{}^\circ C\] becomes triple?
A)
\[546{}^\circ C\]
done
clear
B)
\[182{}^\circ C\]
done
clear
C)
\[819{}^\circ C\]
done
clear
D)
\[646{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 46) If maximum height and range of a projectile are same, what is the angle of projection?
A)
\[30{}^\circ \]
done
clear
B)
\[76{}^\circ \]
done
clear
C)
\[50{}^\circ \]
done
clear
D)
\[90{}^\circ \]
done
clear
View Answer play_arrow
question_answer 47) If wavelength \[\lambda =5400\overset{\text{o}}{\mathop{\text{A}}}\,\] is threshold value for a certain metal, then its work function would be:
A)
2.3 eV
done
clear
B)
0.025 eV
done
clear
C)
10 eV
done
clear
D)
0.23 eV
done
clear
View Answer play_arrow
question_answer 48) If \[\lambda ={{10}^{-10}}\] changes to \[\lambda =0.5\times {{10}^{-10}}\] m, find energy difference \[(\Delta E)\] give to the particle:
A)
\[\Delta E\] is equal to\[\Delta E{{\left( \frac{1}{4} \right)}^{th}}\]of initial energy
done
clear
B)
\[\Delta E\] is equal to \[{{\left( \frac{1}{2} \right)}^{th}}\]of initial energy
done
clear
C)
\[\Delta E\] is equal to twice of initial energy
done
clear
D)
\[\Delta E\] is equal to initial energy
done
clear
View Answer play_arrow
question_answer 49) If inductance of a coil is L and current passing through it is i, find energy stored in it:
A)
\[L{{i}^{2}}\]
done
clear
B)
\[4L{{i}^{2}}\]
done
clear
C)
\[Li\]
done
clear
D)
\[\frac{1}{2}L{{i}^{2}}\]
done
clear
View Answer play_arrow
question_answer 50) A fan is moving around its axis. What will be its motion regarded as?
A)
Pure rolling
done
clear
B)
Rolling with slipping
done
clear
C)
Skidding
done
clear
D)
Pure rotations
done
clear
View Answer play_arrow
question_answer 51) If \[{{a}_{r}}\]and\[{{a}_{t}}\] represent radial and tangential accelerations, the motion of a panicle will be uniformly circular if:
A)
\[{{a}_{r}}=0\]and \[{{a}_{t}}=0\]
done
clear
B)
\[{{a}_{r}}=0\]but\[{{a}_{t}}\ne 0\]
done
clear
C)
\[{{a}_{r}}=0\]but\[{{a}_{t}}=0\]
done
clear
D)
\[{{a}_{r}}\ne 0\]and\[{{a}_{t}}\ne 0\]
done
clear
View Answer play_arrow
question_answer 52) Radius of orbit of satellite of earth is R. Its kinetic energy is proportional to :
A)
\[\frac{1}{R}\]
done
clear
B)
\[\frac{1}{\sqrt{R}}\]
done
clear
C)
\[R\]
done
clear
D)
\[\frac{1}{{{R}^{3/2}}}\]
done
clear
View Answer play_arrow
question_answer 53) The radius R of the soap bubble is doubled under isothermal condition. If T be the surface tension of soap bubble, the required surface energy in doing so is given by :
A)
\[32\pi {{R}^{2}}T\]
done
clear
B)
\[24\pi {{R}^{2}}T\]
done
clear
C)
\[8\pi {{R}^{2}}T\]
done
clear
D)
\[4\pi {{R}^{2}}T\]
done
clear
View Answer play_arrow
question_answer 54) Mercury boils at \[367{}^\circ C.\] However, mercury thermometers are made such that they can measure temperature upto\[500{}^\circ C.\] This is done by:
A)
maintaining vacuum above mercury column in the stem of the thermometer
done
clear
B)
filling nitrogen gas at high pressure above the mercury column
done
clear
C)
filling oxygen gas at high pressure above the mercury column
done
clear
D)
filling nitrogen gas at low pressure above the mercury column
done
clear
View Answer play_arrow
question_answer 55) Two similar coils are kept mutually perpendicular such that their centres coincide. At the centre, find the ratio of the magnetic field due to one coil and the resultant magnetic field through both coils, if the same current is flown:
A)
\[1:\sqrt{2}\]
done
clear
B)
\[1:2\]
done
clear
C)
1: 2
done
clear
D)
\[\sqrt{3}:1\]
done
clear
View Answer play_arrow
question_answer 56) A prism of refractive index \[\sqrt{2}\] has a refracting angle of \[60{}^\circ \]. Ai what angle a ray must be incident on it so that it suffers a minimum deviation?
A)
\[45{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[180{}^\circ \]
done
clear
View Answer play_arrow
question_answer 57) A cone filled with water is revolved in a vertical circle of radius 4 m and the water does not fall down. What must be the maximum period of revolution?
A)
2 s
done
clear
B)
4 s
done
clear
C)
1 s
done
clear
D)
6 s
done
clear
View Answer play_arrow
question_answer 58) A transparent cube of 15 cm edge contains a small air bubble. Its apparent depth when viewed through one face is 6 cm and when viewed through the opposite face is 4 cm. Then the refractive index of the material of the cube is :
A)
2.0
done
clear
B)
2.5
done
clear
C)
1.6
done
clear
D)
1.5
done
clear
View Answer play_arrow
question_answer 59) Light waves travel in vacuum along the y-axis. Which of the following may represent the wave front?
A)
\[y=\]constant
done
clear
B)
\[x=\] constant
done
clear
C)
\[z=\] constant
done
clear
D)
\[x+y+z=\] constant
done
clear
View Answer play_arrow
question_answer 60) A capacitor is connected to a cell of emf E having some internal resistance r. The potential difference across the:
A)
cell is < E
done
clear
B)
cell is E
done
clear
C)
capacitor is > E
done
clear
D)
capacitor is < E
done
clear
View Answer play_arrow
question_answer 61) The length, breadth and thickness of a block are given by \[l\]= 12cm. b = 6 cm and t = 2.45 cm. The volume of the block according 10 the idea of significant figures should be:
A)
\[1\times {{10}^{2}}\,c{{m}^{3}}\]
done
clear
B)
\[2\times {{10}^{2}}\,c{{m}^{3}}\]
done
clear
C)
\[1.763\times {{10}^{2}}\,c{{m}^{3}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 62) Five particles of mass 2 kg are attached to the rim of a circular disc of radius 0.1 m and negligible mass. Moment of inertia of the system about the axis passing through the centre of the disc and perpendicular to its plane is:
A)
\[1\,kg{{m}^{2}}\]
done
clear
B)
\[0.1\,kg{{m}^{2}}\]
done
clear
C)
\[2\,kg{{m}^{2}}\]
done
clear
D)
\[0.2\,kg{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 63) The radius of the convex surface of plano-convex lens is 20 cm and the refractive index of the material of the lens is 1.5. The focal length is:
A)
30 cm
done
clear
B)
50 cm
done
clear
C)
20 cm
done
clear
D)
40 cm
done
clear
View Answer play_arrow
question_answer 64) An ice-cube of density \[900\,kg/{{m}^{3}}\] is floating in water of density \[1000\,kg/{{m}^{3}}\]. The percentage of volume of ice-cube outside the water is:
A)
20%
done
clear
B)
35%
done
clear
C)
10%
done
clear
D)
25%
done
clear
View Answer play_arrow
question_answer 65) A sphere of diameter 0.2 m and mass 2 kg is rolling on an inclined plane with velocity v = 0.5 m/s. The kinetic energy of the sphere is:
A)
0.1 J
done
clear
B)
0.3 J
done
clear
C)
0.5 J
done
clear
D)
0.42 J
done
clear
View Answer play_arrow
question_answer 66) An electron moves at right angle to a magnetic field of \[1.5\times {{10}^{-2}}T\] with a speed of \[6\times {{10}^{7}}\,m/s\]. If the specific charge of the electron is \[1.7\,\times {{10}^{11}}\,C/kg\]. the radius of the circular path will be:
A)
2.9 cm
done
clear
B)
3.9 cm
done
clear
C)
2.35 cm
done
clear
D)
2 cm
done
clear
View Answer play_arrow
question_answer 67) If work function of a metal is 4.2 eV, the cut off wavelength is :
A)
\[8000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[7000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1472\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[2950\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 68) A particle is executing the motion \[x=a\,\cos (\omega t-\theta ).\] The maximum velocity of the particle is :
A)
\[a\,\omega \,\cos \]
done
clear
B)
\[a\,\omega \]
done
clear
C)
\[a\,\omega \,\sin \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 69) A particle is executing two different simple harmonic motions, mutually perpendicular, of different amplitudes and having phase difference of \[\pi /2.\] The path of the particle will be:
A)
circular
done
clear
B)
straight line
done
clear
C)
parabolic
done
clear
D)
elliptical
done
clear
View Answer play_arrow
question_answer 70) Equations of motion in the same direction are given by: \[{{y}_{1}}=2a\,\sin (\omega t-kx)\] \[{{y}_{2}}=2a\,\sin (\omega t-kx-)\] The amplitude of the medium particle will be:
A)
\[2a\,\cos \,\]
done
clear
B)
\[\sqrt{2}a\,\cos \,\]
done
clear
C)
\[4\,a\,\cos \frac{\,}{2}\]
done
clear
D)
\[\sqrt{2}\,a\,\cos \frac{\,}{2}\]
done
clear
View Answer play_arrow
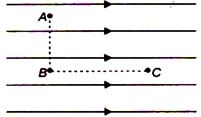
question_answer 71)
Figure shows three points A, B and C in a region of uniform electric field \[\mathbf{\vec{E}}\mathbf{.}\] The line AB is perpendicular and BC is parallel lo the Field lines. Then which of the following holds good?
A)
\[{{V}_{A}}={{V}_{B}}={{V}_{C}}\]
done
clear
B)
\[{{V}_{A}}={{V}_{B}}>{{V}_{C}}\]
done
clear
C)
\[{{V}_{A}}={{V}_{B}}<{{V}_{C}}\]
done
clear
D)
\[{{V}_{A}}>{{V}_{B}}={{V}_{C}}\] where \[{{V}_{A}}>{{V}_{B}}\] Vg and \[{{V}_{C}}\] represent the electric potential at points A. B and C respectively.
done
clear
View Answer play_arrow
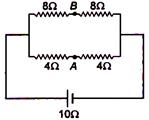
question_answer 72)
The potential difference between points A and B is:
A)
\[\frac{20}{7}V\]
done
clear
B)
\[\frac{40}{7}V\]
done
clear
C)
\[\frac{10}{7}V\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 73) A short linear object of length b lies along the axis of a concave mirror of focal length \[f\] at a distance \[u\] from the pole of mirror, what is the size of image ?
A)
\[\left( \frac{f}{u-f} \right)b\]
done
clear
B)
\[{{\left( \frac{f}{u-f} \right)}^{2}}b\]
done
clear
C)
\[\left( \frac{f}{u-f} \right){{b}^{2}}\]
done
clear
D)
\[\left( \frac{f}{u-f} \right)\]
done
clear
View Answer play_arrow
question_answer 74) A closed organ pipe and an open organ pipe are tuned to the same fundamental frequency. What is the ratio of their lengths?
A)
1 : 2
done
clear
B)
2 : 1
done
clear
C)
2 : 3
done
clear
D)
4 : 3
done
clear
View Answer play_arrow
question_answer 75) Regarding a semiconductor which one of the following is wrong?
A)
There are no free electrons at room temperature
done
clear
B)
There are no free electrons at 0 K
done
clear
C)
The number of free electrons increases with rise of temperature
done
clear
D)
The charge carriers are electrons and holes
done
clear
View Answer play_arrow
question_answer 76) A steel scale measures the length of a copper wire as 80.0 cm, when both are at \[20{}^\circ C\], the calibration temperature for the scale. What would the scale read for the length of the wire when both are ac \[40{}^\circ C\]? Given: \[\alpha \] for steel \[=11\times {{10}^{-6}}{{/}^{\text{o}}}C\] and \[\alpha \] for \[Cu=17\times {{10}^{-6}}\,{{/}^{\text{o}}}C\]:
A)
80.0096 cm
done
clear
B)
80.0272 cm
done
clear
C)
1 cm
done
clear
D)
25.2 cm
done
clear
View Answer play_arrow
question_answer 77) A tank is filled with water upto height H. When a hole is made at a distance h below the level of water, what will be the horizontal range of water jet?
A)
\[2\sqrt{h(H-h)}\]
done
clear
B)
\[4\sqrt{h(H+h)}\]
done
clear
C)
\[4\sqrt{h(H-h)}\]
done
clear
D)
\[2\sqrt{h(H+h)}\]
done
clear
View Answer play_arrow
question_answer 78) A raft of wood of mass 120 kg floats in water. The weight that can be put on the raft to make it just sink, should be : \[({{d}_{raft}}=600\,kg/{{m}^{3}})\]
A)
80 kg
done
clear
B)
50 kg
done
clear
C)
60 kg
done
clear
D)
30 kg
done
clear
View Answer play_arrow
question_answer 79) Nuclear fusion is common to the pair:
A)
thermonuclear reactor, uranium based nuclear reactor
done
clear
B)
energy production in sun, uranium based nuclear reactor
done
clear
C)
energy production in sun hydrogen bomb
done
clear
D)
disintegration of heavy nuclei hydrogen bomb
done
clear
View Answer play_arrow
question_answer 80) Which of the following statements is true for an n-type semi-conductor?
A)
The donor level lies closely below the bottom of the conduction band
done
clear
B)
The donor level lies closely above the top of the valence band
done
clear
C)
The donor level lies at the halfway mark of the forbidden energy gap
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 81) The minimum wavelength of X-rays emitted by X-ray tube is \[0.4125\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. The accelerating voltage is :
A)
30 kV
done
clear
B)
50 kV
done
clear
C)
80 kV
done
clear
D)
60 kV
done
clear
View Answer play_arrow
question_answer 82) A monoatomic gas supplied the heat Q very slowly keeping the pressure constant. The work done by the gas will be :
A)
\[\frac{2}{3}Q\]
done
clear
B)
\[\frac{3}{5}Q\]
done
clear
C)
\[\frac{2}{5}Q\]
done
clear
D)
\[\frac{1}{5}Q\]
done
clear
View Answer play_arrow
question_answer 83)
The refractive index of the material of the prism and liquid are 1.56 and 1.32 respectively. What will be the value of \[\] for the following refraction?
A)
\[\text{sin}\,\ge \frac{13}{11}\]
done
clear
B)
\[\text{sin}\,\ge \frac{11}{13}\]
done
clear
C)
\[\text{sin}\,\ge \frac{\sqrt{3}}{2}\]
done
clear
D)
\[\text{sin}\,\ge \frac{1}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 84) The temperature of the black body increases from T to 2T. The factor by which the rate of emission will increase, is :
A)
4
done
clear
B)
2
done
clear
C)
16
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 85) A police Jeep is chasing with velocity of 45 km/h a thief in another jeep moving with velocity 153 km/h. Police fires a bullet with muzzle velocity of 180 m/s. The velocity with which it will strike the car of the thief is:
A)
150 m/s
done
clear
B)
27 m/s
done
clear
C)
450 m/s
done
clear
D)
250 m/s
done
clear
View Answer play_arrow
question_answer 86) In a sinusoidal wave, the time required, for a particular point to move from maximum displacement to zero displacement is 0.17 s. The frequency of the wave is:
A)
1.47 Hz
done
clear
B)
2.94 Hz
done
clear
C)
0.73 Hz
done
clear
D)
0.36 Hz
done
clear
View Answer play_arrow
question_answer 87) An LC circuit is in the state of resonance. If \[C=0.1\,\pi F\] and \[L=0.25\,H,\] neglecting ohmic resistance of circuit, what is the frequency of
A)
1007 Hz
done
clear
B)
100 Hz
done
clear
C)
109 Hz
done
clear
D)
500 Hz
done
clear
View Answer play_arrow
question_answer 88) A person who can see things most clearly at a distance of 10 cm, requires spectacles to enable to see clearly things at a distance of 30 cm. What should be the focal length of the spectacles?
A)
15 cm (concave)
done
clear
B)
15 cm (convex)
done
clear
C)
10 cm
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 89) The dimensional formula for Youngs modulus is:
A)
\[[M{{L}^{-1}}{{T}^{-2}}]\]
done
clear
B)
\[[{{M}^{0}}L{{T}^{-2}}]\]
done
clear
C)
\[[ML{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 90) When temperature of an ideal gas is increased from \[27{}^\circ C\] to \[227{}^\circ C\], its \[rms\] speed is changed from 400 m/s to \[{{v}_{s}}\] The \[{{v}_{s}}\] is:
A)
516 m/s
done
clear
B)
450 m/s
done
clear
C)
310 m/s
done
clear
D)
746 m/s
done
clear
View Answer play_arrow
question_answer 91) A bar magnet of magnetic moment M is placed in the magnetic held B. The torque acting on the magnet is:
A)
\[\mathbf{\vec{M}\times \vec{B}}\]
done
clear
B)
\[\mathbf{\vec{M}}-\mathbf{\vec{B}}\]
done
clear
C)
\[\frac{1}{2}\mathbf{\vec{M}}\times \mathbf{\vec{B}}\]
done
clear
D)
\[\mathbf{\vec{M}}+\mathbf{\vec{B}}\]
done
clear
View Answer play_arrow
question_answer 92) A capacitor of capacitance \[6\mu F\] is charged upto 100 V. The energy stored in the capacitor is:
A)
0.6 J
done
clear
B)
0.06 J
done
clear
C)
0.03 J
done
clear
D)
0.3 J
done
clear
View Answer play_arrow
question_answer 93) The radius of the orbit of a planet is two rimes that of the earth. The time period of planet is:
A)
4.2 T
done
clear
B)
2.8 T
done
clear
C)
5.6 T
done
clear
D)
8.4 T
done
clear
View Answer play_arrow
question_answer 94) A body falls from a height h = 200 m. The ratio of distance travelled in each 2 s, during t = 0 to t = 6 s of the journey is:
A)
1 : 4 : 9
done
clear
B)
1 : 2 : 4
done
clear
C)
1 : 3 : 5
done
clear
D)
1 : 2 : 3
done
clear
View Answer play_arrow
question_answer 95) To make the frequency double of a spring oscillator, we have to :
A)
reduce the mass to one fourth
done
clear
B)
quardruple the mass
done
clear
C)
double the mass
done
clear
D)
half the mass
done
clear
View Answer play_arrow
question_answer 96) A particle executing SHM has amplitude 0.01 m and frequency 60 Hz. The maximum acceleration of particle is :
A)
\[60{{\pi }^{2\,}}m/{{s}^{2}}\]
done
clear
B)
\[80{{\pi }^{2\,}}m/{{s}^{2}}\]
done
clear
C)
\[120{{\pi }^{2\,}}m/{{s}^{2}}\]
done
clear
D)
\[144{{\pi }^{2\,}}m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 97) When a wave travels in a medium the particles displacement is given by the equation y = 0.03 sin n (2t - 0.01 x), where x and y are in metre and r in second. The wavelength of the wave is :
A)
200 m
done
clear
B)
100 m
done
clear
C)
20 m
done
clear
D)
10 m
done
clear
View Answer play_arrow
question_answer 98) In He-Ne laser the most favorable ratio of helium to neon for satisfactory laser action is :
A)
1 : 4
done
clear
B)
4 : 1
done
clear
C)
1 : 7
done
clear
D)
7 : 1
done
clear
View Answer play_arrow
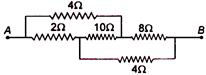
question_answer 99)
Find the equivalent resistance between the points A and B:
A)
\[2\,\Omega \]
done
clear
B)
\[4\,\Omega \]
done
clear
C)
\[8\,\Omega \]
done
clear
D)
\[16\,\Omega \]
done
clear
View Answer play_arrow
question_answer 100) A forward biased diode is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 101) Cadmium rods are used for which purpose?
A)
Emit electrons
done
clear
B)
Absorb neutrons
done
clear
C)
Emit neutrons
done
clear
D)
Absorb electrons
done
clear
View Answer play_arrow
question_answer 102) Propionic acid and \[KOH\] reacts to produce which one of the following?
A)
Potassium propionate
done
clear
B)
Propyl alcohol
done
clear
C)
Propionaldehyde
done
clear
D)
Does not react
done
clear
View Answer play_arrow
question_answer 103) What is the effect of dilution on the equivalent conductance of strong electrolyte?
A)
Decrease on dilution
done
clear
B)
Remains unchanged
done
clear
C)
Increase on dilution
done
clear
D)
None of die above
done
clear
View Answer play_arrow
question_answer 104) \[35.4\,\,mL\] of \[HCl\] is required for the neutralization of a solution containing 0.275 g of sodium hydroxide. The normality of hydrochloric add is?
A)
\[0.97\,\,N\]
done
clear
B)
\[0.142\,\,N\]
done
clear
C)
\[0.194\,\,N\]
done
clear
D)
\[0.244\,\,N\]
done
clear
View Answer play_arrow
question_answer 105) When \[{{H}_{2}}S\] gas is passed in a metal sulphate solution in presence of\[N{{H}_{4}}OH\], a white precipitate is produced. The metal is identified as:
A)
\[Zn\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Pb\]
done
clear
D)
\[Hg\]
done
clear
View Answer play_arrow
question_answer 106) The value of amu is which of the following?
A)
\[1.57\times {{10}^{-24}}kg\]
done
clear
B)
\[1.66\times {{10}^{-24}}kg\]
done
clear
C)
\[1.99\times {{10}^{-23}}kg\]
done
clear
D)
\[1.66\times {{10}^{-27}}kg\]
done
clear
View Answer play_arrow
question_answer 107) The molecule having largest dipole moment among the following is:
A)
\[CH{{I}_{3}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[CC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 108) When calcium acetate is distilled, it will produce which of the following compound?
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 109) Which of the following electronic configuration represents noble gas?
A)
\[n{{s}^{2}}n{{p}^{6}}\]
done
clear
B)
\[n{{s}^{2}}n{{p}^{5}}\]
done
clear
C)
\[n{{s}^{2}}n{{p}^{4}}\]
done
clear
D)
\[n{{s}^{2}}n{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 110) Sodium pyrophosphate is represented by which of the following formula?
A)
\[N{{a}_{2}}P{{O}_{4}}\]
done
clear
B)
\[N{{a}_{4}}{{P}_{2}}{{O}_{5}}\]
done
clear
C)
\[N{{a}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
D)
\[N{{a}_{2}}{{P}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 111) Which one of the following can produce hydrogen when treated with metallic sodium?
A)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 112) Which one of the following has unit positive charge and 1 amu mass?
A)
Electron
done
clear
B)
Neutron
done
clear
C)
Proton
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 113) What is the co-ordination number of body centred cube?
A)
8
done
clear
B)
6
done
clear
C)
4
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 114) Which gas is evolved by the treatment of magnesium with very dilute solution of\[HN{{O}_{3}}?\]
A)
\[{{N}_{2}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 115) Which of the following compound shows aromatic properties?
A)
Valine
done
clear
B)
Leucine
done
clear
C)
Serine
done
clear
D)
Tyrosine
done
clear
View Answer play_arrow
question_answer 116) Which one of the following pair shows Buffers solution?
A)
\[NaCl+NaOH\]
done
clear
B)
\[C{{H}_{3}}COONa+C{{H}_{3}}COOH\]
done
clear
C)
\[C{{H}_{3}}COOH+C{{H}_{3}}COON{{H}_{4}}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}+CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 117) \[PC{{l}_{3}}\] and cold water reacts to produce which of the following?
A)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{2}}\]
done
clear
C)
\[{{H}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
D)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 118) Which of the following converts carbonyl compounds into hydrocarbons?
A)
\[{{H}_{2}}/Pt\]
done
clear
B)
\[LiAl{{H}_{4}}\]
done
clear
C)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[Zn\text{-}Hg/HCl\]
done
clear
View Answer play_arrow
question_answer 119) Which of the following chloride is water insoluble?
A)
\[HCl\]
done
clear
B)
\[AgCl\]
done
clear
C)
Both \[a\] and \[b\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 120) How many neutrons are present in tritium nucleus?
A)
2
done
clear
B)
3
done
clear
C)
1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 121) The total number of protons in \[10\,\,g\] of calcium carbonate is\[({{N}_{0}}=6.023\times {{10}^{23}})\]:
A)
\[3.01\times {{10}^{24}}\]
done
clear
B)
\[4.06\times {{10}^{24}}\]
done
clear
C)
\[2.01\times {{10}^{24}}\]
done
clear
D)
\[3.02\times {{10}^{24}}\]
done
clear
View Answer play_arrow
question_answer 122) By dissolving \[5\,\,g\] substance in \[50\,\,g\] of water, the decrease in freezing point is\[{{1.2}^{o}}C\]. The gram molal depression is\[{{1.85}^{o}}C\]. The molecular weight of substance is:
A)
\[105.4\]
done
clear
B)
\[118.2\]
done
clear
C)
\[137.2\]
done
clear
D)
\[154.2\]
done
clear
View Answer play_arrow
question_answer 123) The high boiling point of water is due to which reason:
A)
co-ordinate bonding
done
clear
B)
convalent bond
done
clear
C)
electrostatic force of attraction
done
clear
D)
hydrogen bonding
done
clear
View Answer play_arrow
question_answer 124) Which is correct for an enothermic reaction?
A)
\[\Delta H\]is positive
done
clear
B)
\[\Delta H\]is negative
done
clear
C)
\[\Delta E\]is negative
done
clear
D)
\[\Delta H=\]zero
done
clear
View Answer play_arrow
question_answer 125) Acetonitriles on hydrolysis produce which of the following?
A)
Amine
done
clear
B)
Acid
done
clear
C)
Amines
done
clear
D)
Carbonyl compounds
done
clear
View Answer play_arrow
question_answer 126) The radius of hydrogen atom is\[0.53\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. The radius of \[_{3}L{{i}^{2+}}\] is of:
A)
\[1.27\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.17\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.57\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[0.99\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 127) The purest form of coal is:
A)
peat
done
clear
B)
anthracite
done
clear
C)
bituminous
done
clear
D)
lignite
done
clear
View Answer play_arrow
question_answer 128) The correct sequence of hybridisation of methane, ethene and acetylene is:
A)
\[sp,\,\,s{{p}^{2}},\,\,s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}},\,\,s{{p}^{3}},\,\,sp\]
done
clear
C)
\[s{{p}^{3}},\,\,s{{p}^{2}},\,\,sp\]
done
clear
D)
\[s{{p}^{3}},\,\,sp,\,\,s{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 129) Which phosphorus reacts with \[KOH\] solution to produce phosphene gas?
A)
White phosphorus
done
clear
B)
Red phosphorus
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 130) Saturated fatty acids are represented by which of the formula?
A)
\[{{C}_{n}}{{H}_{n}}{{O}_{2}}\]
done
clear
B)
\[{{C}_{n}}{{H}_{3n}}{{O}_{2}}\]
done
clear
C)
\[{{C}_{n}}{{H}_{2n+1}}\]
done
clear
D)
\[{{C}_{n}}{{H}_{2n}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 131) \[{{C}_{6}}{{H}_{5}}N{{O}_{2}}\xrightarrow{Sn/HCl}{{C}_{6}}{{H}_{5}}X\] \[X\]is identified as:
A)
\[NO\]
done
clear
B)
\[-N{{H}_{2}}\]
done
clear
C)
\[NHOH\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 132) Acetic acid and \[{{P}_{2}}{{O}_{5}}\] reacts to produce which of the following?
A)
Acetic anhydride
done
clear
B)
Acetaldehyde
done
clear
C)
Phosphoric acid
done
clear
D)
Acetone
done
clear
View Answer play_arrow
question_answer 133) The test for unsaturation is confirmed by the decolourisation of which of the following?
A)
Iodine water
done
clear
B)
\[CuS{{O}_{4}}\]solution
done
clear
C)
Bromine water
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 134) Which of the following element shows maximum valency?
A)
Carbon
done
clear
B)
Barium
done
clear
C)
Nitrogen
done
clear
D)
Sulphur
done
clear
View Answer play_arrow
question_answer 135) The volume of oxygen necessary for the complete combustion of \[20\,\,L\] of propane is:
A)
\[40\,\,L\]
done
clear
B)
\[60\,\,L\]
done
clear
C)
\[80\,\,L\]
done
clear
D)
\[100\,\,L\]
done
clear
View Answer play_arrow
question_answer 136) Which reaction is used for the preparation of acetophenone?
A)
Reimer-Tiemann reaction
done
clear
B)
Wurtz-Fittig reaction
done
clear
C)
Friedel-Crafts reaction
done
clear
D)
Cannizaros reaction
done
clear
View Answer play_arrow
question_answer 137) \[0.005\,\,M\] acid solution has\[5\,\,pH\]. The percentage ionization of acid is:
A)
\[0.8%\]
done
clear
B)
\[0.6%\]
done
clear
C)
\[0.4%\]
done
clear
D)
\[0.2%\]
done
clear
View Answer play_arrow
question_answer 138) PVC polymer can be prepared by which of the monomer?
A)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{2}}=CH-Cl\]
done
clear
View Answer play_arrow
question_answer 139) \[C{{H}_{3}}COOH\] is weaker acid than\[{{H}_{2}}S{{O}_{4}}\]. It is due to:
A)
more ionization
done
clear
B)
less ionization
done
clear
C)
covalent bond
done
clear
D)
electrovalent bond
done
clear
View Answer play_arrow
question_answer 140) The ortho and para hydrogen differ in respect of which of the following?
A)
In the molecular weight
done
clear
B)
In the nature of spin of protons
done
clear
C)
In the nature of spin of electrons
done
clear
D)
In the number of protons
done
clear
View Answer play_arrow
question_answer 141) Which of the following is correct according to adsorption isotherm?
A)
\[\frac{X}{m}\propto p{}^\circ \]
done
clear
B)
\[\frac{X}{m}\propto p\]
done
clear
C)
\[\frac{X}{m}\propto {{p}^{1/n}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 142) \[_{84}R{{n}^{219}}\] is a member of actinium series. The other member of this series is:
A)
\[_{89}A{{c}^{225}}\]
done
clear
B)
\[_{90}T{{h}^{232}}\]
done
clear
C)
\[_{15}{{P}^{35}}\]
done
clear
D)
\[_{92}{{U}^{235}}\]
done
clear
View Answer play_arrow
question_answer 143) The compressibility factor of an ideal gas is:
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[4\]
done
clear
D)
\[zero\]
done
clear
View Answer play_arrow
question_answer 144) Soaps can be classified as:
A)
carbohydrates
done
clear
B)
ether
done
clear
C)
salts of fatty acids
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 145) In a chemical reaction two reactants takes part. The rate of reaction is directly proportional to the concentration of one of them and inversely proportional to the concentration of the other. The order of reaction is:
A)
\[zero\]
done
clear
B)
\[1\]
done
clear
C)
\[2\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 146) A gaseous mixture contains \[56g\] of\[{{N}_{3}}\],\[44g\] of \[C{{O}_{2}}\] and \[16g\] of\[C{{H}_{4}}\]. The total pressure of mixture is \[720\,\,mm\] of\[Hg\]. The partial pressure of methane is:
A)
\[75\,\,atm\]
done
clear
B)
\[160\,\,atm\]
done
clear
C)
\[180\,\,atm\]
done
clear
D)
\[215\,\,atm\]
done
clear
View Answer play_arrow
question_answer 147) The metal used to recover copper from a solution of\[CuS{{O}_{4}}\]is:
A)
\[Fe\]
done
clear
B)
\[He\]
done
clear
C)
\[Na\]
done
clear
D)
\[Ag\]
done
clear
View Answer play_arrow
question_answer 148) Amino acids have peptide linkage which is:
A)
\[-CO-NH-\]
done
clear
B)
\[-C-N{{H}_{2}}\]
done
clear
C)
\[SO-NH-\]
done
clear
D)
\[-CO-N-\]
done
clear
View Answer play_arrow
question_answer 149) Phenacetin is used as:
A)
antipyretics
done
clear
B)
antiseptics
done
clear
C)
analgesic
done
clear
D)
antimalarials
done
clear
View Answer play_arrow
question_answer 150) Gravity separation process is used for the concentration of:
A)
calamine
done
clear
B)
haematite
done
clear
C)
chalcopyrite
done
clear
D)
bauxite
done
clear
View Answer play_arrow
question_answer 151) Which of the following is not an ore of magnesium?
A)
Magnesite
done
clear
B)
Dolomite
done
clear
C)
Gypsum
done
clear
D)
Carnallile
done
clear
View Answer play_arrow
question_answer 152) The solubility of\[S{{b}_{2}}{{S}_{3}}\], in water is \[1.0\times {{10}^{-5}}\] \[mol/L\] at\[298\,\,K\]. What will be its solubility product?
A)
\[108\times {{10}^{-25}}\]
done
clear
B)
\[1.0\times {{10}^{-25}}\]
done
clear
C)
\[144\times {{10}^{-25}}\]
done
clear
D)
\[126\times {{10}^{-24}}\]
done
clear
View Answer play_arrow
question_answer 153) What will be the \[pH\] value of \[0.05\,\,M\]\[Ba{{(OH)}_{2}}\] solution?
A)
\[12\]
done
clear
B)
\[13\]
done
clear
C)
\[1\]
done
clear
D)
\[12.96\]
done
clear
View Answer play_arrow
question_answer 154) Which of the following will not react with\[NaOH\]?
A)
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
D)
\[CH{{(CN)}_{3}}\]
done
clear
View Answer play_arrow
question_answer 155) Industrial name for\[{{H}_{2}}{{S}_{2}}{{O}_{7}}\]is:
A)
Pyrosulphuric acid
done
clear
B)
Marshalls acid
done
clear
C)
Oleum
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 156) \[{{I}_{2}}\]dissolves in \[K\,\,I\] solution due to the formation of:
A)
\[K{{I}_{2}}\]and\[{{I}^{-}}\]
done
clear
B)
\[{{K}^{+}},\,\,{{I}^{-}}\]and\[{{I}_{2}}\]
done
clear
C)
\[I_{3}^{-}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 157) Water gas is:
A)
\[CO+{{N}_{2}}\]
done
clear
B)
\[CO+C{{O}_{2}}+C{{H}_{4}}\]
done
clear
C)
\[C{{O}_{2}}+{{N}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 158) Chloroform, when kept open. is oxidized to:
A)
\[C{{O}_{2}}\]
done
clear
B)
\[COC{{l}_{2}}\]
done
clear
C)
\[C{{O}_{2}},\,\,C{{l}_{2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 159) Iodoform test is not given by:
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 160) In Me Arthur Forest Method, silver is extracted from the solution of b\[Na[Ag{{(CN)}_{2}}]\] by the use of:
A)
\[Fe\]
done
clear
B)
\[Mg\]
done
clear
C)
\[Cu\]
done
clear
D)
\[Zn\]
done
clear
View Answer play_arrow
question_answer 161) \[IUPAC\]name of: \[C{{H}_{3}}C{{H}_{2}}C(Br)=CH-Cl\]is:
A)
2-bromo-1-chtoro butene
done
clear
B)
1-chloro-2-bromo butene
done
clear
C)
3-chioro-2-bromo butene-2
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 162) Which of the following elements never show positive oxidation number?
A)
\[O\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Ga\]
done
clear
D)
\[F\]
done
clear
View Answer play_arrow
question_answer 163) Which of the following is anhydride of perchloric acid?
A)
\[C{{l}_{2}}{{O}_{7}}\]
done
clear
B)
\[C{{l}_{2}}{{O}_{5}}\]
done
clear
C)
\[C{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[HClO\]
done
clear
View Answer play_arrow
question_answer 164) Quantitative measurement of nitrogen in an organic compound is done by the method:
A)
Berthelot method
done
clear
B)
Belstein method
done
clear
C)
Lassaigne test
done
clear
D)
Kjheldahl method
done
clear
View Answer play_arrow
question_answer 165) Benedicts solution is not reduced by :
A)
formaldehyde
done
clear
B)
acetatdehyde
done
clear
C)
glucose
done
clear
D)
acetic anhydride
done
clear
View Answer play_arrow
question_answer 166) Which of the following sulphides is yellow in colour?
A)
\[Cus\]
done
clear
B)
\[Cds\]
done
clear
C)
\[ZnS\]
done
clear
D)
\[CoS\]
done
clear
View Answer play_arrow
question_answer 167) Which of the following element is a metalloid?
A)
\[Bi\]
done
clear
B)
\[Sn\]
done
clear
C)
\[Ge\]
done
clear
D)
\[C\]
done
clear
View Answer play_arrow
question_answer 168) Brown ring in the test of \[NO_{3}^{-}\] is formed due to the formation of:
A)
\[FeS{{O}_{4}}\cdot NO\]
done
clear
B)
\[[Fe{{(S{{O}_{4}})}_{2}}\cdot NO]{{H}_{2}}O\]
done
clear
C)
\[F{{e}_{2}}{{(S{{O}_{4}})}_{3}}\cdot NO\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 169) The vapour pressure will be lowest for:
A)
\[0.1\,\,M\]sugar solution
done
clear
B)
\[0.1\,\,M\,\,KCl\] solution
done
clear
C)
\[0.1\,\,M\,\,Cu{{(N{{O}_{3}})}_{2}}\] solution
done
clear
D)
\[0.1\,\,M\,\,AgN{{O}_{3}}\]solution
done
clear
View Answer play_arrow
question_answer 170) Which of the following is a false statement?
A)
Fluorine is more electronegative than chlorine
done
clear
B)
Nitrogen has greater \[I{{E}_{1}}\] than oxygen
done
clear
C)
Lithium is amphoteric
done
clear
D)
Chlorine is an oxidizing agent
done
clear
View Answer play_arrow
question_answer 171) What is the name of element with atomic number\[105\]?
A)
Kurchatovium
done
clear
B)
Dubnium
done
clear
C)
Nobelium
done
clear
D)
Holmium
done
clear
View Answer play_arrow
question_answer 172) Which kind of fission is favoured by sunlight?
A)
Heterolytic fission
done
clear
B)
Homolytic fission
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 173) The reagent used in Gatterman Koch aldehyde synthesis is:
A)
\[Pb/BaS{{O}_{4}}\]
done
clear
B)
alkaline\[KMn{{O}_{4}}\]
done
clear
C)
acidic\[KMn{{O}_{4}}\]
done
clear
D)
\[CO+HCl\]
done
clear
View Answer play_arrow
question_answer 174) Which type of isomerism is shown by propanal and propanone?
A)
Functional group
done
clear
B)
Metamerism
done
clear
C)
Tautomerism
done
clear
D)
Chain isomerism
done
clear
View Answer play_arrow
question_answer 175) lionization depends upon:
A)
pressure
done
clear
B)
volume
done
clear
C)
dilution
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 176) Which of the following oxide does not form acidic aqueous solution?
A)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
D)
\[NO\]
done
clear
View Answer play_arrow
question_answer 177) Which of the following is a use of alum?
A)
Making explosives
done
clear
B)
Bleaching clothes
done
clear
C)
Water softening
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 178) The first ionization potential is maximum for:
A)
\[B\]
done
clear
B)
\[N\]
done
clear
C)
\[O\]
done
clear
D)
\[Be\]
done
clear
View Answer play_arrow
question_answer 179) Which of the following salt does not get hydrolyzed in water?
A)
\[KCl{{O}_{4}}\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[C{{H}_{3}}COONa\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 180) In the reaction:\[{{H}_{2}}+{{I}_{2}}=2HI\] In a \[2\,\,L\] flask 0.4 moles of each \[{{H}_{2}}\] and \[{{I}_{2}}\] are taken. At equilibrium 0.5 moles of \[HI\] are formed. What will be the value of equilibrium constant\[{{K}_{c}}\]?
A)
\[20.2\]
done
clear
B)
\[25.4\]
done
clear
C)
\[0.284\]
done
clear
D)
\[11.1\]
done
clear
View Answer play_arrow
question_answer 181) Blood cells will remain as such in:
A)
hypertonic solution
done
clear
B)
hypotonic solution
done
clear
C)
isotonic solution
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 182) Which among the following elements have lowest value of\[I{{E}_{1}}\]?
A)
\[Pb\]
done
clear
B)
\[Sn\]
done
clear
C)
\[Si\]
done
clear
D)
\[C\]
done
clear
View Answer play_arrow
question_answer 183) \[KI\]and \[CuS{{O}_{4}}\] solution when mixed, give:
A)
\[Cu{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{u}_{2}}{{I}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}+C{{u}_{2}}{{I}_{2}}+{{I}_{2}}\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}+Cu{{I}_{2}}+{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 184) Which of the following is a Lewis base?
A)
\[NaOH\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[BC{{l}_{3}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 185) Wave nature of electrons was demonstrated by:
A)
Schrodinger
done
clear
B)
De-Broglie
done
clear
C)
Davisson and Gamier
done
clear
D)
Heisenberg
done
clear
View Answer play_arrow
question_answer 186) Distribution law was given by:
A)
Henry
done
clear
B)
Vant Hoff
done
clear
C)
Nernst
done
clear
D)
Ostwald
done
clear
View Answer play_arrow
question_answer 187) When \[Cu\] reacts with \[AgN{{O}_{3}}\] solution, the reaction takes place is:
A)
oxidation of\[Cu\]
done
clear
B)
reduction of\[Cu\]
done
clear
C)
oxidation of\[Ag\]
done
clear
D)
reduction of\[NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 188) Cetane is a compound which has very good ignition property. Chemically it is:
A)
\[C{{H}_{3}}{{(C{{H}_{2}})}_{14}}C{{H}_{3}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}C{{(C{{H}_{2}})}_{11}}C{{H}_{3}}\]
done
clear
C)
\[{{C}_{17}}{{H}_{34}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 189) Aldol condensation will not occur in:
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 190) Which of the following alcohol is used as beverage?
A)
Propanol
done
clear
B)
2-butanol
done
clear
C)
Methanol
done
clear
D)
Ethanol
done
clear
View Answer play_arrow
question_answer 191) The oxidation number of carbon in \[C{{H}_{2}}O\] is:
A)
\[-2\]
done
clear
B)
\[+2\]
done
clear
C)
\[0\]
done
clear
D)
\[+4\]
done
clear
View Answer play_arrow
question_answer 192) Which of the following reaction involves oxidation and reduction?
A)
\[NaBr+HCl\xrightarrow{{}}NaCl+HBr\]
done
clear
B)
\[HBr+AgN{{O}_{3}}\xrightarrow{{}}AgBr+HN{{O}_{3}}\]
done
clear
C)
\[{{H}_{2}}+B{{r}_{2}}\xrightarrow{{}}2HBr\]
done
clear
D)
\[N{{a}_{2}}O+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 193) The electrophile involved in the nitration of benzene is:
A)
\[N{{O}_{2}}\]
done
clear
B)
\[NO_{2}^{+}\]
done
clear
C)
\[NO\]
done
clear
D)
\[NO_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 194)
The correct order of ease of dehydration of following is:
A)
\[I>II>III\]
done
clear
B)
\[III>II>I\]
done
clear
C)
\[I>III>II\]
done
clear
D)
\[III>I>II\]
done
clear
View Answer play_arrow
question_answer 195) Fluorine is the best oxidizing agent because it has:
A)
highest electron affinity
done
clear
B)
highest\[E_{red}^{\text{o}}\]
done
clear
C)
highest\[E_{oxid}^{\text{o}}\]
done
clear
D)
lowest electron affinity
done
clear
View Answer play_arrow
question_answer 196) The bond order of \[O_{2}^{+}\] is the same as in:
A)
\[N_{2}^{+}\]
done
clear
B)
\[C{{N}^{-}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[N{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 197) When acetamide reacts with Bra and caustic soda, then we get:
A)
acetic acid
done
clear
B)
bromoaceric acid
done
clear
C)
methyl amine
done
clear
D)
ethyl amine
done
clear
View Answer play_arrow
question_answer 198) Bleaching action of \[S{{O}_{2}}\] is due to its:
A)
oxidizing property
done
clear
B)
acidic property
done
clear
C)
basic property
done
clear
D)
reducing property
done
clear
View Answer play_arrow
question_answer 199) When \[{{I}_{2}}\] is passed through \[KCl,\,\,KF\] and\[KBr\]solutions:
A)
\[C{{l}_{2}}\]and \[B{{r}_{2}}\] are evolved
done
clear
B)
\[C{{l}_{2}}\] is evolved
done
clear
C)
\[C{{l}_{2}},\,\,B{{r}_{2}}\]and \[{{F}_{2}}\] are evolved
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 200) Which of the following dissolves in hot cone. \[NaOH\]solution?
A)
\[Fe\]
done
clear
B)
\[Zn\]
done
clear
C)
\[Cn\]
done
clear
D)
\[Ag\]
done
clear
View Answer play_arrow
question_answer 201) The adipose tissue in the skin is found in the:
A)
dermis
done
clear
B)
subcutaneous layer
done
clear
C)
Malpighian layer
done
clear
D)
epidermal layer
done
clear
View Answer play_arrow
question_answer 202) In horses, rabbits, hares, the cellulose gets digested in the :
A)
caecum
done
clear
B)
stomach
done
clear
C)
appendix
done
clear
D)
rumen
done
clear
View Answer play_arrow
question_answer 203) The process of breathing is controlled by
A)
spinal cord
done
clear
B)
cerebellum
done
clear
C)
medulla oblongata
done
clear
D)
hypothalamus
done
clear
View Answer play_arrow
question_answer 204) A person with type A blood group may safely receive a transfusion of :
A)
type AB
done
clear
B)
type A and type O
done
clear
C)
type A and type AB
done
clear
D)
done
clear
View Answer play_arrow
question_answer 205) If a man who is colour blind marries a woman who is pure normal for colour vision, the chances of their sons having colour blindness is :
A)
100%
done
clear
B)
50 : 50
done
clear
C)
0% (zero)
done
clear
D)
75 : 25
done
clear
View Answer play_arrow
question_answer 206) Brachydactyly is due to :
A)
dominant gene on the autosome
done
clear
B)
recessive gene on the aulosomc
done
clear
C)
dominant gene on the sex chromosome
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 207) The cause of Cat-cry syndrome is due to:
A)
loss of a segment of X-chromosome
done
clear
B)
loss of a segment of 5th chromosome
done
clear
C)
loss of segment of Y-chromosome
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 208) Lateral roots are originated from :
A)
cortex
done
clear
B)
pericycle
done
clear
C)
endodermis
done
clear
D)
pith
done
clear
View Answer play_arrow
question_answer 209) What will be the sequence of nucleorides in m-RNA if DNA have ATTGGC sequence?
A)
TAAGGG
done
clear
B)
UGATCA
done
clear
C)
ATAGCG
done
clear
D)
UAACCG
done
clear
View Answer play_arrow
question_answer 210) Parasexuality is involved with:
A)
fusion of gamete and protoplast
done
clear
B)
fusion of male gamete with secondary nucleus
done
clear
C)
fusion of protoplast
done
clear
D)
fusion of male and female gamete
done
clear
View Answer play_arrow
question_answer 211) How many types of gametes may be produced by genotype D/d: E/e:F/f ?
A)
27
done
clear
B)
8
done
clear
C)
3
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 212) In which of the following pyrenoids are present?
A)
Marchantia
done
clear
B)
Riccia
done
clear
C)
Anthoceros
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 213) Liquid part of coconut is called:
A)
male gametophyte
done
clear
B)
nucellus
done
clear
C)
endosperm
done
clear
D)
embryo
done
clear
View Answer play_arrow
question_answer 214) Kaziranga is associated with :
A)
Rhinoceros
done
clear
B)
tigers
done
clear
C)
birds
done
clear
D)
lions
done
clear
View Answer play_arrow
question_answer 215) The red colour of red sea is due to which of the following blue-green algae?
A)
Chlamydomonas nivalis
done
clear
B)
Anabaena
done
clear
C)
Microcystis
done
clear
D)
Trichodesmium
done
clear
View Answer play_arrow
question_answer 216) The function of restriction enzyme is :
A)
cleavages of protein
done
clear
B)
cleavages of DNA
done
clear
C)
cleavages of fat
done
clear
D)
cleavages of starch
done
clear
View Answer play_arrow
question_answer 217) Cystolith is made up of:
A)
calcium oxide
done
clear
B)
calcium oxalate
done
clear
C)
calcium carbonate
done
clear
D)
calcium chloride
done
clear
View Answer play_arrow
question_answer 218) The algae used in space research is :
A)
Cephaleuros
done
clear
B)
Gelidium
done
clear
C)
Chlorella
done
clear
D)
Gracilaria
done
clear
View Answer play_arrow
question_answer 219) Mendel was not imagine about which of the following?
A)
Linkage
done
clear
B)
Incomplete dominance
done
clear
C)
Dominance
done
clear
D)
Segregation of alleles
done
clear
View Answer play_arrow
question_answer 220) Sunnhemp is obtained from :
A)
Crotolaria juncea
done
clear
B)
Linum usitatiseimum
done
clear
C)
Corchorus capsularis
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 221) The enzyme involved in Fixation of \[C{{O}_{2}}\] in \[{{C}_{4}}\] plants is:
A)
PEP carboxylase
done
clear
B)
RuDP carboxylase
done
clear
C)
RuDP oxydase
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 222) Which of the following follows Chargaff rule?
A)
\[\text{A}\,\text{+}\,\text{G}\,\text{=}\,\text{T}\,\text{+}\,\text{C}\]
done
clear
B)
\[\text{A}\,\,\text{=}\,\,\text{C}\]
done
clear
C)
\[G\,\,\text{=}\,\,T\]
done
clear
D)
\[\frac{\text{A+T}}{\text{G}\,\text{+}\,\text{C}}\text{=1}\]
done
clear
View Answer play_arrow
question_answer 223) The endosperm of gymnosperm is :
A)
haploid
done
clear
B)
diploid
done
clear
C)
triploid
done
clear
D)
tetraploid
done
clear
View Answer play_arrow
question_answer 224) Which type of movement is present in Mimosa pudica?
A)
Nycnastic
done
clear
B)
Seismonasric
done
clear
C)
Chemonastic
done
clear
D)
Phototrophic
done
clear
View Answer play_arrow
question_answer 225) Respiratory roots and viviparous reproduction are the characteristic of:
A)
hydrophytes
done
clear
B)
xerophytes
done
clear
C)
mesophytes
done
clear
D)
mangroves
done
clear
View Answer play_arrow
question_answer 226) Amphivasal vascular bundle possess :
A)
xylem around phloem
done
clear
B)
phloem around xylem
done
clear
C)
phloem on both sides of xylem
done
clear
D)
phloem towards centre and xylem towards periphery
done
clear
View Answer play_arrow
question_answer 227) During development of embryo in archegonium of Bryophyta its posterior part form protective embryo cover which is called :
A)
calyptra
done
clear
B)
paraphysis
done
clear
C)
apophysis
done
clear
D)
hypophysis
done
clear
View Answer play_arrow
question_answer 228) Which of the following is the example of structural protein?
A)
Myosin
done
clear
B)
Collagen
done
clear
C)
Keratin
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 229) Infectious stage of Plasmodium is :
A)
ookinece
done
clear
B)
sporozoite
done
clear
C)
oocyst
done
clear
D)
gametocyts
done
clear
View Answer play_arrow
question_answer 230) Nematoblast of Hydra are :
A)
sensory
done
clear
B)
complicated
done
clear
C)
with nematocyst apparatus
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 231) In Krebs cycle formation of:
A)
34 ATP takes place
done
clear
B)
38 ATP takes place
done
clear
C)
15 ATP from each acetyl Co-A takes place
done
clear
D)
12 ATP from each acetyl Co-A takes place
done
clear
View Answer play_arrow
question_answer 232) Which of the protozoan is responsible for kala azar disease?
A)
Trypanosoma
done
clear
B)
Giardia
done
clear
C)
Monocystis
done
clear
D)
Leishmania
done
clear
View Answer play_arrow
question_answer 233) Hardest part in animal body is :
A)
bone
done
clear
B)
hair
done
clear
C)
dentine
done
clear
D)
enamel
done
clear
View Answer play_arrow
question_answer 234) In which direction cristae of rabbit ear helps in maintaining balance?
A)
Circular position of longitudinal axis of semicircular canals
done
clear
B)
Transverse position of longitudinal axis of semicircular canals
done
clear
C)
Parallel to longitudinal axis of semicircular canals
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 235) Function of thyroxin hormone is :
A)
to grow
done
clear
B)
to develop
done
clear
C)
self immunization
done
clear
D)
to control metabolism
done
clear
View Answer play_arrow
question_answer 236) The number of chromosomes becomes half in :
A)
anaphase-\[\text{I}\]
done
clear
B)
anaphase-\[\text{II}\]
done
clear
C)
telophase-\[\text{I}\]
done
clear
D)
telophase-\[\text{H}\]
done
clear
View Answer play_arrow
question_answer 237) \[{{O}_{2}}\] does not evolved in photosynthesis of :
A)
BGA
done
clear
B)
green algae
done
clear
C)
bacteria
done
clear
D)
autotrophic plant
done
clear
View Answer play_arrow
question_answer 238) Stratification occurs in:
A)
desert
done
clear
B)
tropical forest
done
clear
C)
deciduous forest
done
clear
D)
tundra
done
clear
View Answer play_arrow
question_answer 239) Heroin is :
A)
diacetyl morphine
done
clear
B)
triacetyl morphine
done
clear
C)
letra acetyl morphine
done
clear
D)
mono acetyl morphine
done
clear
View Answer play_arrow
question_answer 240) Conditions helpful in photo respiration are :
A)
more \[{{O}_{2}}\] and less \[C{{O}_{2}}\]
done
clear
B)
less \[{{O}_{4}}\] and more \[C{{O}_{3}}\]
done
clear
C)
more temperature and less \[{{O}_{2}}\]
done
clear
D)
more humidity and less temperature
done
clear
View Answer play_arrow
question_answer 241) Which of the following is the result of double fertilization?
A)
Cotyledon
done
clear
B)
Nucellus
done
clear
C)
Endosperm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 242) Who discovered the super bugs?
A)
H.G. Khorana
done
clear
B)
Dilip Sah
done
clear
C)
Anand Mohan Chakarwarty
done
clear
D)
Robert Hooke
done
clear
View Answer play_arrow
question_answer 243) Book lungs are the respiratory organs in :
A)
protozoans
done
clear
B)
cnidarians
done
clear
C)
arthropods
done
clear
D)
amphibians
done
clear
View Answer play_arrow
question_answer 244) Which is the function of nor-epinephrin?
A)
Increase blood pressure
done
clear
B)
Urine formation
done
clear
C)
Increase secretion of adrenalin
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 245) 45. Phenomenon of industrial melanism demonstrates :
A)
reproductive isolation
done
clear
B)
genetic isolation
done
clear
C)
natural selection
done
clear
D)
geographical isolation
done
clear
View Answer play_arrow
question_answer 246) Primary acceptor of \[C{{O}_{2}}\] in photosynthesis is:
A)
phosphoric acid
done
clear
B)
ribulose phosphate
done
clear
C)
glucose
done
clear
D)
ribulose 1, 5 biphosphate
done
clear
View Answer play_arrow
question_answer 247) In the following how the sap wood is converted into heart wood?
A)
By degeneration of protoplast of living cells
done
clear
B)
Tylosis formation
done
clear
C)
By deposition of resins, oil, gums
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 248) The difference in phloem of gymnosperms and angiosperms is due to :
A)
parenchyma
done
clear
B)
sieve cell
done
clear
C)
companion cell
done
clear
D)
fibres
done
clear
View Answer play_arrow
question_answer 249) When tall and dwarf plants are crossed, from which cross 1 : 1 ratio is obtained?
A)
Tt and tt
done
clear
B)
tt and tt
done
clear
C)
\[\text{Tt}\,\text{ }\!\!\times\!\!\text{ }\,\text{Tt}\]
done
clear
D)
TT and Tt
done
clear
View Answer play_arrow
question_answer 250) Botanical name of species which cause white rust of crucifers?
A)
Peronospora parasitica
done
clear
B)
Puccinia gramnis
done
clear
C)
Pythium debarganum
done
clear
D)
Albugo Candida
done
clear
View Answer play_arrow
question_answer 251) Root cap is absent in :
A)
mesophyics
done
clear
B)
hydrophytes
done
clear
C)
epiphytes
done
clear
D)
xerophyies
done
clear
View Answer play_arrow
question_answer 252) Which type of rhizoids are present in Riccia?
A)
Unicellular smooth
done
clear
B)
Multicellular smooth
done
clear
C)
Unicellular smooth and tuberculated
done
clear
D)
Multicellular smooth and tuberculated
done
clear
View Answer play_arrow
question_answer 253) In Mirabilis a hybrid for red (RR) and white (rr) flower produces pink (R-r) flower. A plant with pink flower is crossed with white flower the expected phenotypic ratio is :
A)
red : pink : white (1 : 2 : 1)
done
clear
B)
pink : white (1 : 1)
done
clear
C)
red : pink (1 : 1)
done
clear
D)
red .-white (3 : 1)
done
clear
View Answer play_arrow
question_answer 254) Which of the following is the site of lipid synthesis?
A)
Rough ER
done
clear
B)
Smooth ER
done
clear
C)
Golgi bodies
done
clear
D)
Ribosome
done
clear
View Answer play_arrow
question_answer 255) Cilliaced epithelium is present in;
A)
trachea
done
clear
B)
ureter
done
clear
C)
intestine
done
clear
D)
nasal chamber
done
clear
View Answer play_arrow
question_answer 256) Pulp cavity of teeth is lined by :
A)
odontoblast
done
clear
B)
chondroblast
done
clear
C)
osteoblast
done
clear
D)
amyloblast
done
clear
View Answer play_arrow
question_answer 257) Which of the following is not a source of vitamin-A?
A)
Carrot
done
clear
B)
Mango
done
clear
C)
Apple
done
clear
D)
Yeast
done
clear
View Answer play_arrow
question_answer 258) Complete metamorphosis is found in :
A)
house-fly and mosquito
done
clear
B)
house-fly and cockroach
done
clear
C)
mosquito and cockroach
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 259) Down syndrome is due to :
A)
chromosome number increase in 21st pair autosome
done
clear
B)
chromosome number decrease in 21st pair autosome
done
clear
C)
chromosome number increase in 18th pair autosome
done
clear
D)
chromosome number decrease in 18th air autosome
done
clear
View Answer play_arrow
question_answer 260) Colour blindness is due to:
A)
recessive female chromosome
done
clear
B)
dominant female chromosome
done
clear
C)
recessive male chromosome
done
clear
D)
dominant male chromosome
done
clear
View Answer play_arrow
question_answer 261) Genetic recombination is due to:
A)
fertilization and meiosis
done
clear
B)
mitosis and meiosis
done
clear
C)
fertilization and mitosis
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 262) Legume plants are important for atmosphere because they :
A)
help in \[N{{O}_{2}}\] fixation
done
clear
B)
do not help in \[N{{O}_{3}}\] fixation
done
clear
C)
increase soil fertility
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 263) Community is:
A)
a group of independent and interacting population of different species
done
clear
B)
a group of independent and interacting population of same species
done
clear
C)
a group of independent and interacting population of same species in a specific area
done
clear
D)
a group of independent and interacting population of different species in a specific area
done
clear
View Answer play_arrow
question_answer 264) Quiescent centre is present in:
A)
shoot apex
done
clear
B)
root apex
done
clear
C)
both (a) and (b)
done
clear
D)
meristemacic tissue
done
clear
View Answer play_arrow
question_answer 265) Connecting link between reptiles and birds is :
A)
Dodo
done
clear
B)
Dimetrodon
done
clear
C)
Sphenodon
done
clear
D)
Archaeopleryx
done
clear
View Answer play_arrow
question_answer 266) Vestigial organ in human being is :
A)
incisor
done
clear
B)
canines
done
clear
C)
molar
done
clear
D)
premolar
done
clear
View Answer play_arrow
question_answer 267) Miller and Urey performed an experiment to prove the origin of life. They took gases \[N{{H}_{3}}\] and \[{{H}_{2}}\] along with :
A)
\[{{N}_{2}}\] and \[{{H}_{2}}O\]
done
clear
B)
\[{{H}_{2}}O\] and \[C{{H}_{4}}\]
done
clear
C)
\[C{{H}_{4}}\] and \[{{N}_{2}}\]
done
clear
D)
\[C{{O}_{2}}\] and \[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 268) Nerve impulse initiates with the movements of:
A)
\[{{K}^{+}}\]
done
clear
B)
\[N{{a}^{+}}\]
done
clear
C)
\[C{{a}^{+}}\]
done
clear
D)
\[M{{g}^{+}}\]
done
clear
View Answer play_arrow
question_answer 269) In which of the following, Nissls granules are found?
A)
Liver cells
done
clear
B)
Nerve cells
done
clear
C)
Intestinal cells
done
clear
D)
Uriniferous tubules
done
clear
View Answer play_arrow
question_answer 270) Sympathetic nervous system induces :
A)
heart beat
done
clear
B)
secretion of semen
done
clear
C)
secretion of saliva
done
clear
D)
secretion of digestive juices
done
clear
View Answer play_arrow
question_answer 271) Organ of corti is found in:
A)
heart
done
clear
B)
kidneys
done
clear
C)
inner ear
done
clear
D)
nasal chamber
done
clear
View Answer play_arrow
question_answer 272) During food chain, maximum energy is stored in :
A)
producer
done
clear
B)
primary consumer
done
clear
C)
secondary consumer
done
clear
D)
decomposer
done
clear
View Answer play_arrow
question_answer 273) Cold treatment of seeds is called :
A)
verbalization
done
clear
B)
stratification
done
clear
C)
devemalization
done
clear
D)
photophosphorylation
done
clear
View Answer play_arrow
question_answer 274) One gene-one enzyme hypothesis was given by:
A)
Mendel
done
clear
B)
Darwin
done
clear
C)
Robert Hooke
done
clear
D)
Beadle and Tatum
done
clear
View Answer play_arrow
question_answer 275) The formation of fruits without fertilization is called :
A)
polyspory
done
clear
B)
parthenogenesis
done
clear
C)
polyembryony
done
clear
D)
parthenocarpy
done
clear
View Answer play_arrow
question_answer 276) Mycorrhiza helps in :
A)
respiration
done
clear
B)
water absorption
done
clear
C)
nutrient absorption
done
clear
D)
phosphate absorption
done
clear
View Answer play_arrow
question_answer 277) Last electron receptor in respiration is :
A)
\[C{{O}_{2}}\]
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}\]
done
clear
D)
NADH
done
clear
View Answer play_arrow
question_answer 278) Which of the following algae is parasitic?
A)
Nostoc
done
clear
B)
Rhodomenia
done
clear
C)
Cephaleuros
done
clear
D)
Polysiphoma
done
clear
View Answer play_arrow
question_answer 279) Alcoholic fermentation takes place in the presence of:
A)
maltase
done
clear
B)
zymase
done
clear
C)
amylase
done
clear
D)
invertase
done
clear
View Answer play_arrow
question_answer 280) Fluid mosaic model of cell membrane was given by:
A)
Beadle and Tatum
done
clear
B)
Singer and Nicolson
done
clear
C)
Watson and Crick
done
clear
D)
Robenson and Miller
done
clear
View Answer play_arrow
question_answer 281) Suicidal bags are :
A)
lysosomes
done
clear
B)
Golgi bodies
done
clear
C)
ribosomes
done
clear
D)
chloroplast
done
clear
View Answer play_arrow
question_answer 282) Heterochromatin remains condensed in which part of chromosome?
A)
Secondary construction-I
done
clear
B)
Secondary construction-II
done
clear
C)
Telomeres
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 283) Tracheids differs from vessels in having :
A)
thick wall
done
clear
B)
bordered pits
done
clear
C)
discontinuous intercalary wall
done
clear
D)
spiral thickening
done
clear
View Answer play_arrow
question_answer 284) Chlorosis in leaf occurs due to the deficiency of:
A)
chloride
done
clear
B)
magnesium
done
clear
C)
phosphate
done
clear
D)
molybdenum
done
clear
View Answer play_arrow
question_answer 285) The anti transpirant is :
A)
PMA
done
clear
B)
ABA
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 286) Pneumatophores are found in :
A)
Orchid
done
clear
B)
Piper
done
clear
C)
Rhizophora
done
clear
D)
Ficus
done
clear
View Answer play_arrow
question_answer 287) Plant which used by Hugo de Vries for mutation experiment was :
A)
Oenothera lamarkiana
done
clear
B)
Solarium tuberosom
done
clear
C)
Ficus elastic
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 288) Agar-agar is obtained from :
A)
Chlorealla
done
clear
B)
Chlamydomonas
done
clear
C)
Nostoc
done
clear
D)
Gracilaria and Gelidium
done
clear
View Answer play_arrow
question_answer 289) Hypanthodium in florescence is found in :
A)
Ficus
done
clear
B)
Tulsi
done
clear
C)
Cedrus
done
clear
D)
Calotropis
done
clear
View Answer play_arrow
question_answer 290) Green-house gases are :
A)
\[CFCS,\,C{{O}_{2}},\,N{{H}_{4}}\], and \[N{{O}_{2}}\]
done
clear
B)
\[{{O}_{2}},\,{{N}_{3}},\,N{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}},\,C{{O}_{2}},\,N{{H}_{4}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 291) Respiration pigment of blood in cockroach is :
A)
haemozoine
done
clear
B)
haemocyanin
done
clear
C)
haemoglobin
done
clear
D)
absent
done
clear
View Answer play_arrow
question_answer 292) From acrosome which secrets :
A)
hyaluronic acid
done
clear
B)
hyaluronidase
done
clear
C)
TSH
done
clear
D)
fertilizing
done
clear
View Answer play_arrow
question_answer 293) Malignant malaria is cause by :
A)
Plasmadium falciparum
done
clear
B)
Plasmodium ovale
done
clear
C)
Plasmodium vivax
done
clear
D)
Plasmodium malariae
done
clear
View Answer play_arrow
question_answer 294) Retrogressive metamorphosis is present in
A)
Herdmania
done
clear
B)
Balanoglossus
done
clear
C)
Amphioxus
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 295) Corpus leuteum secrets :
A)
LH
done
clear
B)
progesterone
done
clear
C)
estrogen
done
clear
D)
FSH
done
clear
View Answer play_arrow
question_answer 296) Origin of nervous system from :
A)
meso-endoderm
done
clear
B)
mesoderm
done
clear
C)
endoderm
done
clear
D)
ectoderm
done
clear
View Answer play_arrow
question_answer 297) Which parasite is present in seminal vesicle of earthworm?
A)
Monocystu
done
clear
B)
Nosema
done
clear
C)
Sarcocystis
done
clear
D)
Nictotherus
done
clear
View Answer play_arrow
question_answer 298) Ribonucleoprotein panicles of protoplasm are:
A)
ribosomes
done
clear
B)
plastid
done
clear
C)
Golgi body
done
clear
D)
cristae
done
clear
View Answer play_arrow
question_answer 299) Main function of ribosomes is :
A)
fat synthesis
done
clear
B)
sterol synthesis
done
clear
C)
protein synthesis
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 300) 100. Which of the following is shift in chloride- shift?
A)
\[{{O}_{2}}\] and \[C{{O}_{2}}\]
done
clear
B)
Bicarbonate ions
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow