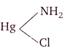
question_answer 1)
The value of current J in the circuit will be
A)
1.7 A
done
clear
B)
2.1 A
done
clear
C)
3 A
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 2) Two plane mirrors are inclined to each other at angle \[60{}^\circ \]. Ray incident on first mirror parallel to the second mirror becomes parallel to first mirror after reflection. Angle of deviation is
A)
\[30{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[120{}^\circ \]
done
clear
View Answer play_arrow
question_answer 3)
The equivalent capacitance of the circuit will be
A)
\[\text{1}\text{F}\]
done
clear
B)
\[\text{3}\text{F}\]
done
clear
C)
\[\text{9}\text{F}\]
done
clear
D)
\[\text{4}\text{F}\]
done
clear
View Answer play_arrow
question_answer 4)
If the equivalent capacitance between A and B is \[\text{1}\text{F,}\]then the value of C will be
A)
\[\text{2}\text{F}\]
done
clear
B)
\[\text{4}\text{F}\]
done
clear
C)
\[\text{3}\text{F}\]
done
clear
D)
\[\text{6}\text{F}\]
done
clear
View Answer play_arrow
question_answer 5)
If the input is given between A and C, then the output at the ends of R will be
A)
fully rectified
done
clear
B)
half rectified
done
clear
C)
AC
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 6)
If the acceleration-displacement graph of simple harmonic motion of a particle is given, then the time period of the particle will be
A)
\[\text{2}\pi \]
done
clear
B)
\[\text{3}\pi \]
done
clear
C)
\[4\pi \]
done
clear
D)
\[\text{5}\pi \]
done
clear
View Answer play_arrow
question_answer 7)
Figure shows four, p-V diagram for the given sample of gas. In which case no exchange of heat occurs with the sample?
A)
P
done
clear
B)
Q
done
clear
C)
R
done
clear
D)
S
done
clear
View Answer play_arrow
question_answer 8) The charge on a particle Y is double the charge on particle X. These two particles X and Y after being accelerated through the same potential difference enter a region of uniform magnetic field and describe circular paths of radii R1 and R2 respectively. The ratio of the mass of X to that of Y is
A)
\[{{(2{{R}_{1}}/{{R}_{2}})}^{2}}\]
done
clear
B)
\[{{({{R}_{1}}/2{{R}_{2}})}^{2}}\]
done
clear
C)
\[R_{1}^{2}/2R_{2}^{2}\]
done
clear
D)
\[2{{R}_{1}}/{{R}_{2}}\]
done
clear
View Answer play_arrow
question_answer 9) In a step up transformer the turn ratio is 1 : 10. A resistance of\[200\,\Omega \]connected across the secondary is drawing a current of 0.5 A. What is the primary voltage and current?
A)
50 V, 1 A
done
clear
B)
10 V, 5 A
done
clear
C)
25 V, 4 A
done
clear
D)
20 V, 2 A
done
clear
View Answer play_arrow
question_answer 10) Dimensions of \[{{\varepsilon }_{0}}\] are
A)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{\text{-1}}}{{\text{T}}^{\text{2}}}\text{A }\!\!]\!\!\text{ }\]
done
clear
B)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{2}}{{\text{L}}^{\text{-3}}}{{\text{T}}^{\text{2}}}\text{A }\!\!]\!\!\text{ }\]
done
clear
C)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{-1}}{{\text{L}}^{\text{-3}}}{{\text{T}}^{\text{4}}}{{\text{A}}^{2}}\text{ }\!\!]\!\!\text{ }\]
done
clear
D)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{2}}{{\text{L}}^{\text{3}}}{{\text{T}}^{\text{-2}}}\text{A }\!\!]\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 11) Dimensions of \[\frac{R}{L}\]are
A)
\[[{{T}^{2}}]\]
done
clear
B)
\[[T]\]
done
clear
C)
\[[{{T}^{-1}}]\]
done
clear
D)
\[[{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 12) The unit of magnetic moment is
A)
\[A-{{m}^{2}}\]
done
clear
B)
A-m
done
clear
C)
\[A-{{m}^{3}}\]
done
clear
D)
\[kg-{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 13) In SI system the unit of dipole moment is
A)
C-m
done
clear
B)
\[C-{{m}^{2}}\]
done
clear
C)
\[C/{{m}^{2}}\]
done
clear
D)
C/m
done
clear
View Answer play_arrow
question_answer 14) Electron volt is a unit of
A)
potential
done
clear
B)
charge
done
clear
C)
power
done
clear
D)
energy
done
clear
View Answer play_arrow
question_answer 15) Which of the following can be written as\[\text{kg-}{{\text{m}}^{\text{2}}}{{\text{A}}^{\text{-2}}}{{\text{T}}^{\text{-2}}}\text{?}\]
A)
Inductance
done
clear
B)
Reactance
done
clear
C)
Capacitance
done
clear
D)
Resistance
done
clear
View Answer play_arrow
question_answer 16) If the frequency of\[{{K}_{\alpha ,}}\] X-ray of the element of atomic number 31 is\[f,\] then the frequency of\[{{K}_{\alpha ,}}\] X-ray for atomic number 51 is
A)
25/9 \[f\]
done
clear
B)
16/25\[f\]
done
clear
C)
9/25 \[f\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 17) A bird weighing 1 kg sitting on the base of a wire mesh cage weighing 1.5 kg. The bird starts flying in the cage. The weight of the bird cage assembly will be
A)
1250g
done
clear
B)
1500g
done
clear
C)
1750g
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 18) If the escape velocity for a monkey from the earth surface is 11.2 km/s, then the escape velocity for the elephant is
A)
less than 11.2
done
clear
B)
more than 11.2
done
clear
C)
11.2 km/s
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 19) If the sphere of iron is heated, then its
A)
density decreases
done
clear
B)
volume increases
done
clear
C)
radius decreases
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 20) If a bar magnet is droping through the copper ring, then its velocity
A)
decreases
done
clear
B)
increases
done
clear
C)
remain unaffected
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 21) The power factor of a good choke coil is
A)
nearly zero
done
clear
B)
exactly zero
done
clear
C)
nearly one
done
clear
D)
exactly one
done
clear
View Answer play_arrow
question_answer 22) If two spheres of same masses and radius are brought in contact, then the force of attraction between them will be proportional to
A)
\[{{r}^{2}}\]
done
clear
B)
\[{{r}^{3}}\]
done
clear
C)
\[{{r}^{6}}\]
done
clear
D)
\[{{r}^{4}}\]
done
clear
View Answer play_arrow
question_answer 23) Capacitors are used for
A)
AC
done
clear
B)
DC
done
clear
C)
Both AC and DC
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 24) The ratio of current flowing through primary and secondary of a transformers depends on
A)
number of turns
done
clear
B)
resistance
done
clear
C)
capacitance
done
clear
D)
inductance
done
clear
View Answer play_arrow
question_answer 25) A proton, a deuteron and an a-particle having the same momentum, enters a region of uniform electric field between the parallel plates of a capacitor. If the electric field is perpendicular to the initial direction of the particles. Then, the ratio of deviations of these particles
A)
1 : 2 : 8
done
clear
B)
1 : 2 : 4
done
clear
C)
1 : 2 : 2
done
clear
D)
1 : 1 : 2
done
clear
View Answer play_arrow
question_answer 26) If a radioactive substance decays \[\frac{\text{1}}{\text{16}}\text{th}\] of its original amount in 2 h, then the half-life of that substance is
A)
15 min
done
clear
B)
30 min
done
clear
C)
45 min
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 27) If on applying the potential of 20 V on a conductor its conductance becomes\[8\,{{(\Omega )}^{-1}},\] then the current flowing through it will be
A)
120 A
done
clear
B)
160 A
done
clear
C)
90 A
done
clear
D)
80 A
done
clear
View Answer play_arrow
question_answer 28)
Equivalent resistance of the given circuit is
A)
R
done
clear
B)
R/2
done
clear
C)
R/4
done
clear
D)
R/6
done
clear
View Answer play_arrow
question_answer 29) In a biprism experiment by using light of wavelength \[5000\overset{\text{o}}{\mathop{\text{A}}}\,,\] 5 mm wide fringes are obtained on a screen 1.0 m away from the coherent sources. The separation between the two coherent sources is
A)
1.0 mm
done
clear
B)
0.1 mm
done
clear
C)
0.05 mm
done
clear
D)
0.01 mm
done
clear
View Answer play_arrow
question_answer 30) The power of lens used by a short-sighted person is -2 D. Find the maximum distance of an object, which he can see without spectacles
A)
25 cm
done
clear
B)
50 cm
done
clear
C)
100 cm
done
clear
D)
10 cm
done
clear
View Answer play_arrow
question_answer 31) Kirchhoffs first law \[(\Sigma i=0)\] expresses the conservation of
A)
energy
done
clear
B)
charge
done
clear
C)
momentum
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 32) In p-type semiconductors majority charge carriers are
A)
electrons
done
clear
B)
holes
done
clear
C)
neutrons
done
clear
D)
protons
done
clear
View Answer play_arrow
question_answer 33) A \[1\,\mu F\] capacitance of TV is subjected to 4000 V potential difference. The energy stored in the capacitor is
A)
8 J
done
clear
B)
16 J
done
clear
C)
\[4\times {{10}^{-3}}\,J\]
done
clear
D)
\[2\times {{10}^{-3}}\,J\]
done
clear
View Answer play_arrow
question_answer 34) A body moves a distance of 10 m along a straight line under the action of a force of 5 N. If the work done is 25 J the angle, which the force makes with the direction of motion of the body is
A)
\[0{}^\circ \]
done
clear
B)
\[30{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[90{}^\circ \]
done
clear
View Answer play_arrow
question_answer 35) A particle of mass m is projected with a velocity v making an angle of \[45{}^\circ \] with the horizontal. The magnitude of angular momentum of the projectile about the axis of projection, when the particle is at maximum height
A)
zero
done
clear
B)
\[\frac{m{{v}^{3}}}{4\sqrt{2g}}\]
done
clear
C)
\[\frac{m{{v}^{3}}}{\sqrt{2g}}\]
done
clear
D)
\[\frac{m{{v}^{2}}}{2g}\]
done
clear
View Answer play_arrow
question_answer 36) If the moment of inertia of a disc about an axis tangential and parallel to its surface be\[l,\]then what will be the moment of inertia about the axis tangential but perpendicular to the surface?
A)
\[\frac{6}{5}I\]
done
clear
B)
\[\frac{3}{4}I\]
done
clear
C)
\[\frac{3}{2}I\]
done
clear
D)
\[\frac{5}{4}I\]
done
clear
View Answer play_arrow
question_answer 37) The magnetic needle of a tangent galvanometer is deflected by an angle \[30{}^\circ \] due to a magnet. The horizontal component of earths magnetic field is \[0.34\,\times {{10}^{-4}}T\] along the plane of the coil. The intensity of magnetic field of magnet is
A)
\[1.96\,\times {{10}^{-4}}T\]
done
clear
B)
\[1.96\,\times {{10}^{-5}}T\]
done
clear
C)
\[1.96\,\times {{10}^{4}}T\]
done
clear
D)
\[1.96\,\times {{10}^{5}}T\]
done
clear
View Answer play_arrow
question_answer 38) A coil of resistance R and inductance L is connected to a battery of emf E volt. The final current in the coil is
A)
\[\text{E/R}\]
done
clear
B)
\[\text{E/L}\]
done
clear
C)
\[\sqrt{E/({{R}^{2}}+{{L}^{2}})}\]
done
clear
D)
\[\sqrt{\frac{EL}{({{R}^{2}}+{{L}^{2}})}}\]
done
clear
View Answer play_arrow
question_answer 39) The refractive indices of violet and red light are 1.54 and 1.52 respectively. If the angle of the prism is \[10{}^\circ \], the angular dispersion is
A)
\[0.02{}^\circ \]
done
clear
B)
\[0.2{}^\circ \]
done
clear
C)
\[3.06{}^\circ \]
done
clear
D)
\[30.6{}^\circ \]
done
clear
View Answer play_arrow
question_answer 40) The focal lengths of the objective and eye-piece of a telescope are respectively 100 cm and 2 cm. The moon substends an angle of \[0.5{}^\circ \] at the eye. If it is looked through the telescope, the angle subtended by the moons image will be
A)
\[100{}^\circ \]
done
clear
B)
\[50{}^\circ \]
done
clear
C)
\[25{}^\circ \]
done
clear
D)
\[10{}^\circ \]
done
clear
View Answer play_arrow
question_answer 41) The temperature of a body on Kelvin scale is found to be x K. When it is measured by Fahrenheit thermometer, it is found to be\[x{}^\circ F\], then the value of x is
A)
30
done
clear
B)
313
done
clear
C)
574.25
done
clear
D)
301.25
done
clear
View Answer play_arrow
question_answer 42) 42. Two containers of equal volume contain the same gas at pressure\[{{P}_{1}}\]and\[{{P}_{2}}\]and absolute temperature\[{{T}_{1}}\]and\[{{T}_{2}}\]respectively. On joining the vessels the gas reaches a common pressure p and common temperature T. The ratio p/T is equal to
A)
\[\frac{{{P}_{1}}}{{{T}_{1}}}+\frac{{{P}_{2}}}{{{T}_{2}}}\]
done
clear
B)
\[\frac{{{p}_{1}}{{T}_{1}}+{{p}_{2}}{{T}_{2}}}{{{({{T}_{1}}+{{T}_{2}})}^{2}}}\]
done
clear
C)
\[\frac{{{p}_{1}}{{T}_{2}}+{{p}_{2}}{{T}_{1}}}{{{({{T}_{1}}+{{T}_{2}})}^{2}}}\]
done
clear
D)
\[\frac{{{p}_{1}}}{2{{T}_{1}}}\,+\frac{{{p}_{2}}}{2{{T}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 43) \[1\,c{{m}^{3}}\] of water at its boiling point absorbs 540 cal of heat to become steam with a volume of \[1671\,c{{m}^{3}}\]. If the atmospheric pressure \[=1.013\times {{10}^{5}}\,N/{{m}^{2}}\] and the mechanical equivalent of heat= 4.19J/cal, the energy spent in this process in overcoming intermolecular forces is
A)
540 cal
done
clear
B)
40 cal
done
clear
C)
500 cal
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 44) N atoms of a radioactive element emit n-alpha particles per second. The half-life of the element is
A)
\[\frac{n}{N}s\]
done
clear
B)
\[\frac{N}{n}s\]
done
clear
C)
\[\frac{0.693N}{n}s\]
done
clear
D)
\[\frac{0.693n}{N}s\]
done
clear
View Answer play_arrow
question_answer 45) Two long parallel wires P and Q are both perpendicular to the plane of the paper with distance 5 m between them. If P and Q carry current of 2.5 A and 5 A respectively in the same direction, then the magnetic field at a point half way between the wires is
A)
\[\frac{\sqrt{3}{{\mu }_{0}}}{2\pi }\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{\pi }\]
done
clear
C)
\[\frac{3{{\mu }_{0}}}{2\pi }\]
done
clear
D)
\[\frac{{{\mu }_{0}}}{2\pi }\]
done
clear
View Answer play_arrow
question_answer 46) An alternating potential of frequency\[f\]is applied on a circuit containing a resistance R and a choke L in series. The impedance of this current is
A)
\[R+2\pi fL\]
done
clear
B)
\[\sqrt{{{R}^{2}}+4{{\pi }^{2}}{{f}^{2}}{{L}^{2}}}\]
done
clear
C)
\[\sqrt{{{R}^{2}}+{{L}^{2}}}\]
done
clear
D)
\[\sqrt{{{R}^{2}}+2\pi fL}\]
done
clear
View Answer play_arrow
question_answer 47) There is a voltmeter in a circuit. In order to triple its range, the resistance of how much value should be used?
A)
2R
done
clear
B)
R/2
done
clear
C)
3R
done
clear
D)
4R
done
clear
View Answer play_arrow
question_answer 48)
A wire is bent in the form of a triangle now the equivalent resistance R between its one end and the midpoint of the side is
A)
\[\frac{5R}{12}\]
done
clear
B)
\[\frac{7R}{12}\]
done
clear
C)
\[\frac{3R}{12}\]
done
clear
D)
\[\frac{R}{12}\]
done
clear
View Answer play_arrow
question_answer 49) Which of the following is incorrect?
A)
\[{{C}_{V}}=\frac{R}{\gamma -1}\]
done
clear
B)
\[{{C}_{P}}=\frac{\gamma R}{\gamma -1}\]
done
clear
C)
\[\frac{{{C}_{P}}}{{{C}_{V}}}=\gamma \]
done
clear
D)
\[{{C}_{P}}-{{C}_{V}}=2R\]
done
clear
View Answer play_arrow
question_answer 50) A mass of 20 kg moving with a speed of 10 m/s collides with another stationary mass of 5 kg. As a result of the collision both masses stick together. The kinetic energy of the composite mass will be
A)
600 J
done
clear
B)
800 J
done
clear
C)
1000 J
done
clear
D)
1200 J
done
clear
View Answer play_arrow
question_answer 51) The electric field between two parallel plates of a capacitor is \[2.1\,\times {{10}^{-5}}\]. If a medium is inserted between the plates than the electric field becomes \[1.0\,\times {{10}^{-5}}\]. Now, the value of dielectric will be
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 52) A ray of light propagates from glass (refractive index = 3/2) to water (refractive index = 4/3). The value of the critical angle is
A)
\[{{\sin }^{-1}}\left( \frac{1}{2} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \sqrt{\frac{8}{9}} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{8}{9} \right)\]
done
clear
D)
\[{{\sin }^{-1}}\left( \frac{5}{7} \right)\]
done
clear
View Answer play_arrow
question_answer 53) An electric heater of resistance\[6\,\Omega \] is run for 10 min on a 120 V line. The energy liberated in this period of time is
A)
\[7.2\,\times {{10}^{3}}J\]
done
clear
B)
\[14.4\,\times {{10}^{5}}J\]
done
clear
C)
\[43.2\,\times {{10}^{4}}J\]
done
clear
D)
\[28.8\,\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 54) Lenzs law gives
A)
the magnitude of the induced emf
done
clear
B)
the direction of the induced current
done
clear
C)
both the magnitude and direction of the induced current
done
clear
D)
the magnitude of the induced current
done
clear
View Answer play_arrow
question_answer 55) When two bodies collide elastically, then
A)
kinetic energy of the system alone is conserved
done
clear
B)
only momentum is conserved
done
clear
C)
both energy and momentum are conserved
done
clear
D)
neither energy nor momentum is conserved
done
clear
View Answer play_arrow
question_answer 56) A satellite moves around the earth in a circular orbit of radius r with speed v. If the mass of the satellite is M, its total energy is
A)
\[-1/2M_{v}^{2}\]
done
clear
B)
\[1/2M_{v}^{2}\]
done
clear
C)
\[3/2M_{v}^{2}\]
done
clear
D)
\[M_{v}^{2}\]
done
clear
View Answer play_arrow
question_answer 57) A telescope, consisting of an objective of focal length 60 cm and a single eye lens of focal length 5 cm is of cussed on a distant object is such a way that parallel rays comes out from the eye lens. If the object subtends an angle \[2{}^\circ \] at the objective the angle subtended by the image at the eye is
A)
\[10{}^\circ \]
done
clear
B)
\[24{}^\circ \]
done
clear
C)
\[50{}^\circ \]
done
clear
D)
\[1/6{}^\circ \]
done
clear
View Answer play_arrow
question_answer 58) If the steel bob of a simple pendulum is replaced by a wooden bob, then its time period will
A)
increase
done
clear
B)
decrease
done
clear
C)
remains the same
done
clear
D)
first increases and then decreases
done
clear
View Answer play_arrow
question_answer 59) An electric dipole is placed along the x-axis at the origin 0. A point P is at a distance of 20 cm from this origin such that OP makes an angle\[\text{/3}\]with the x-axis. If the electric field at P makes an angle \[\theta \] with the x-axis, the value of \[\theta \] would be
A)
\[\frac{\pi }{3}\]
done
clear
B)
\[\frac{\pi }{3}+{{\tan }^{-1}}\left( \frac{\sqrt{3}}{2} \right)\]
done
clear
C)
\[\frac{2\pi }{3}\]
done
clear
D)
\[{{\tan }^{-1}}\left( \frac{\sqrt{3}}{2} \right)\]
done
clear
View Answer play_arrow
question_answer 60) The wavelength of light in two liquids x and y are \[3500\overset{\text{o}}{\mathop{\text{A}}}\,\] and \[7000\overset{\text{o}}{\mathop{\text{A}}}\,\], then the critical angle of x relative to y will be
A)
\[60{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[30{}^\circ \]
done
clear
D)
\[15{}^\circ \]
done
clear
View Answer play_arrow
question_answer 61) An ideal transformer has 500 rums in the primary and 2500 in the secondary. The meters of the secondary are indicating 200 V, 8 A, under these conditions. What would the meters of the primary read?
A)
100 V, 16 A
done
clear
B)
40 V, 40 A
done
clear
C)
160 V, 10 A
done
clear
D)
80 V, 20 A
done
clear
View Answer play_arrow
question_answer 62) Sensitivity of potentiometer can be increased by
A)
increasing the emf of the cell
done
clear
B)
increasing the length of the potentiometer wire
done
clear
C)
decreasing the length of the potentiometer wire
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 63) The following four wires are made of same material and same tension is applied on them. Which one will have maximum increase in length?
A)
Length = 100 cm, Diameter = 1 mm
done
clear
B)
Length = 50 cm, Diameter = 0.5 mm
done
clear
C)
Length = 200 cm, Diameter = 2 mm
done
clear
D)
Length = 300 cm, Diameter = 3 mm
done
clear
View Answer play_arrow
question_answer 64) Radius of a capillary is \[2\times {{10}^{-3}}m\]. A liquid of weight \[6.28\,\times {{10}^{-4}}\,N\] may remain in the capillary, then the surface tension of liquid will be
A)
\[5\times {{10}^{-3}}\,N/m\]
done
clear
B)
\[5\times {{10}^{-2}}\,N/m\]
done
clear
C)
5 N/m
done
clear
D)
50 N/m
done
clear
View Answer play_arrow
question_answer 65) If two tuning forks A and B are sounded together, they produce 4 beats/s. A is then slightly loaded with wax, they produce 2 beats/s when sounded again. The frequency of A is 256 Hz. The frequency of B will be
A)
250 Hz
done
clear
B)
252 Hz
done
clear
C)
260 Hz
done
clear
D)
262 Hz
done
clear
View Answer play_arrow
question_answer 66) Two waves are given by\[=a\sin (\omega t-kx)\]and \[{{y}_{2}}=a\cos (\omega t-kx).\]The phase difference between the two waves is
A)
\[\text{/4}\]
done
clear
B)
\[\]
done
clear
C)
\[\text{/8}\]
done
clear
D)
\[\text{/2}\]
done
clear
View Answer play_arrow
question_answer 67) Period of oscillation of mass attached to a spring and performing SHM is T. The spring is now cut into four equal pieces and the same mass attached to one piece. Now, the period of its simple harmonic oscillations is
A)
2T
done
clear
B)
T/4
done
clear
C)
T/2
done
clear
D)
T
done
clear
View Answer play_arrow
question_answer 68) An electron makes a transition from orbit n = 4 to the orbit n = 2 of a hydrogen atom. The wave number of the emitted radiation (R = Rydbergs constant) will be
A)
16/3R
done
clear
B)
2R/16
done
clear
C)
3R/16
done
clear
D)
4R/16
done
clear
View Answer play_arrow
question_answer 69) A uniform rope of length \[l\] lies on a table. If the coefficient of friction is \[\mu \] then the maximum length \[{{l}_{1}}\] of the part of this rope which can overhang from the edge of the table without sliding down is
A)
\[\frac{l}{\mu }\]
done
clear
B)
\[\frac{l}{\mu +1}\]
done
clear
C)
\[\frac{\mu l}{1+\mu }\]
done
clear
D)
\[\frac{\mu l}{\mu -1}\]
done
clear
View Answer play_arrow
question_answer 70) The magnetic flux linked with a coil at any instant t is given by \[\phi =5{{t}^{3}}\,-100t+300,\] the emf induced in the coil after t = 2 s is
A)
- 40 V
done
clear
B)
40 V
done
clear
C)
140 V
done
clear
D)
300 V
done
clear
View Answer play_arrow
question_answer 71) An ideal heat engine working between temperature\[{{T}_{1}}\] and \[{{T}_{2}}\]has an efficiency \[\eta \] the new efficiency if temperature of both the source and sink are doubled, will be
A)
\[\text{/2}\]
done
clear
B)
\[\]
done
clear
C)
\[2\,\]
done
clear
D)
\[\text{3}\,\]
done
clear
View Answer play_arrow
question_answer 72) A 50 g bullet moving with velocity 10 m/s strikes a block of mass 950 g at rest and gets embedded into it. The loss in kinetic energy will be
A)
100%
done
clear
B)
95%
done
clear
C)
5%
done
clear
D)
50%
done
clear
View Answer play_arrow
question_answer 73) The sun radiates energy in all directions. The average radiation received on the earth surface from the sun per second is \[1.4\,kW/{{m}^{3}}\]. The average distance between sun and earth is \[1.5\times {{10}^{11}}m\]. The mass lost by the sun per day
A)
\[4.4\,\times {{10}^{10}}kg\]
done
clear
B)
\[7.6\,\times {{10}^{14}}kg\]
done
clear
C)
\[3.8\times {{10}^{11}}kg\]
done
clear
D)
\[3.8\times {{10}^{14}}kg\]
done
clear
View Answer play_arrow
question_answer 74) Every gas behaves as an ideal gas
A)
at high temperature and low pressure
done
clear
B)
at low temperature and high pressure
done
clear
C)
at normal temperature and pressure
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 75) In an AC circuit, the current is given by\[I=5\,\sin \,(100t-\pi /2)\] and the alternating potential is \[V=200\,\sin (100)t\] volt. The power consumed in the circuit is
A)
20 W
done
clear
B)
40 W
done
clear
C)
1000 W
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 76) The maximum wavelength of radiation that can produce photoelectric effect in a certain metal is 200 nm. The maximum kinetic energy acquired by electron due to radiation of wavelength 100 nm will be
A)
12.4 eV
done
clear
B)
6.2 eV
done
clear
C)
100 eV
done
clear
D)
200 eV
done
clear
View Answer play_arrow
question_answer 77) The material of wire of potentiometer is
A)
copper
done
clear
B)
steel
done
clear
C)
manganin
done
clear
D)
aluminium
done
clear
View Answer play_arrow
question_answer 78) A liquid cools down from \[70{}^\circ C\] to \[60{}^\circ C\] in 5 min. The time taken to cool it from \[60{}^\circ C\] to \[50{}^\circ C\] will be
A)
5 min
done
clear
B)
lesser than 5 min
done
clear
C)
greater than 5 min
done
clear
D)
lesser or greater than 5 min depending upon the density of the liquid
done
clear
View Answer play_arrow
question_answer 79) There are 50 turns of a wire in every cm length of a long solenoid. If 4 A current is flowing in the solenoid, the approximate value of magnetic field along its axis at an internal point and at one end will be respectively
A)
\[\begin{align} & \text{12}\text{.6 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}}}\text{Wb/}{{\text{m}}^{\text{2}}} \\ & \text{6}\text{.3 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}}}\text{Wb/}{{\text{m}}^{\text{2}}} \\ \end{align}\]
done
clear
B)
\[\begin{align} & \text{12}\text{.6 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}}}\text{Wb/}{{\text{m}}^{\text{2}}} \\ & 25.1\times {{10}^{-3}}Wb/{{m}^{2}} \\ \end{align}\]
done
clear
C)
\[\begin{align} & \text{25}\text{.1 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}}}\text{Wb/}{{\text{m}}^{\text{2}}} \\ & \text{12}\text{.6 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}}}\text{Wb/}{{\text{m}}^{\text{2}}} \\ \end{align}\]
done
clear
D)
\[\begin{align} & \text{25}\text{.1 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-5}}}\text{Wb/}{{\text{m}}^{\text{2}}} \\ & \text{12}\text{.6 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-5}}}\text{Wb/}{{\text{m}}^{\text{2}}} \\ \end{align}\]
done
clear
View Answer play_arrow
question_answer 80) Monochromatic light of frequency \[5\times {{10}^{14}}Hz\] travelling in vacuum enters medium of refractive index 1.5. Its wavelength in the medium is
A)
\[4000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[5000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[6000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[5500\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 81) The vector form of Biot-Savarts law for a current carrying element is
A)
\[d\mathbf{\vec{B}}=\frac{{{\mu }_{0}}}{4\pi }\frac{Id\mathbf{\vec{1}}\,\sin \,\phi }{{{r}^{2}}}\]
done
clear
B)
\[\mathbf{\vec{B}}=\frac{{{\mu }_{0}}}{4\pi }\frac{Idl\,\mathbf{\vec{r}}}{{{r}^{2}}}\]
done
clear
C)
\[d\mathbf{\vec{B}}=\frac{{{\mu }_{0}}}{4\pi }\frac{Id\mathbf{\vec{1}}\times \,\mathbf{\vec{r}}}{{{r}^{3}}}\]
done
clear
D)
\[d\,\mathbf{\vec{B}}=\frac{{{\mu }_{0}}}{4\pi }\frac{Id\mathbf{\vec{1}}\times \,\mathbf{\vec{r}}}{{{r}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 82) The potential energy of a body is given by \[U=A-B{{x}^{2}}\] (where x is the displacement). The magnitude of force acting on the particle is
A)
constant
done
clear
B)
proportional to x
done
clear
C)
proportional to \[{{x}^{2}}\]
done
clear
D)
proportional to 1/x
done
clear
View Answer play_arrow
question_answer 83) Spherical ball of radius R is falling in a viscous fluid of viscosity \[\eta \]with a velocity v. The retarding viscous force acting on the spherical ball is
A)
directly proportional to radius R but inversely proportional to velocity v
done
clear
B)
directly proportional to both radius R and to velocity v
done
clear
C)
inversely proportional to both radius R and velocity v
done
clear
D)
inversely proportional to radius R but directly proportional to velocity v
done
clear
View Answer play_arrow
question_answer 84) Even Carnot engine cannot give 100% efficiency because we cannot
A)
prevent radiation
done
clear
B)
find ideal sources
done
clear
C)
reach absolute zero temperature
done
clear
D)
eliminate friction
done
clear
View Answer play_arrow
question_answer 85) The work function of a substance is 4.0 eV. The longest wavelength of light that can cause photoelectron emission from this substance is approximately
A)
540 nm
done
clear
B)
400 nm
done
clear
C)
310 nm
done
clear
D)
220 nm
done
clear
View Answer play_arrow
question_answer 86) A particle of charge \[-16\,\times {{10}^{-18}}C\] moving with velocity \[10\,m{{s}^{-1}}\] along the \[x\]-axis enters a region where a magnetic field of induction B is along the y-axis and an electric field of magnitude \[\text{1}{{\text{0}}^{\text{4}}}\text{V/m}\] is along the negative z-axis. If the charged particle continues moving along the \[x\]-axis, the magnitude of B is
A)
\[{{10}^{16}}\,Wb/{{m}^{2}}\]
done
clear
B)
\[{{10}^{5}}\,Wb/{{m}^{2}}\]
done
clear
C)
\[{{10}^{3}}\,Wb/{{m}^{2}}\]
done
clear
D)
\[{{10}^{-3}}\,Wb/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 87) If \[{{N}_{0}}\] is the original mass of the substance of half-life period \[{{T}_{1/2}}\,=5\,yr,\] then the amount of substance left after 15 yr is
A)
\[{{N}_{0}}/8\]
done
clear
B)
\[{{N}_{0}}/16\]
done
clear
C)
\[{{N}_{0}}/2\]
done
clear
D)
\[{{N}_{0}}/4\]
done
clear
View Answer play_arrow
question_answer 88)
A charged ball B hangs from a silk thread S, which makes an angle \[\theta \] with a large charged conducting sheet P, as shown in the figure. The surface charge density \[\sigma \] of the sheet is proportional to
A)
cot \[\theta \]
done
clear
B)
cot \[\theta \]
done
clear
C)
tan \[\theta \]
done
clear
D)
sin \[\theta \]
done
clear
View Answer play_arrow
question_answer 89) If\[{{\theta }_{i}}\]is the inversion temperature,\[{{\theta }_{n}}\]is the neutral temperature, \[{{\theta }_{c}}\]is the temperature of the cold junction for thermocouple, then
A)
\[{{\theta }_{i}}+{{\theta }_{c}}={{\theta }_{n}}\]
done
clear
B)
\[{{\theta }_{i}}-{{\theta }_{c}}=2{{\theta }_{n}}\]
done
clear
C)
\[\frac{{{\theta }_{i}}+{{\theta }_{c}}}{2}\theta n\]
done
clear
D)
\[{{\theta }_{c}}-{{\theta }_{i}}=2{{\theta }_{n}}\]
done
clear
View Answer play_arrow
question_answer 90) Speeds of two identical cars are u are 4u at a specific instant. The ratio of the respective distances in which the two cars are stopped in the same time
A)
1 : 1
done
clear
B)
1 : 4
done
clear
C)
1 : 8
done
clear
D)
1 : 16
done
clear
View Answer play_arrow
question_answer 91) Before using the tangent galvanometer, its coil is set up in
A)
magnetic meridian
done
clear
B)
perpendicular to magnetic meridian
done
clear
C)
at angle of \[45{}^\circ \] to magnetic meridian
done
clear
D)
it does not require any setting
done
clear
View Answer play_arrow
question_answer 92)
Which of the following plots may represent reactance of a series LC combination?
A)
a
done
clear
B)
b
done
clear
C)
c
done
clear
D)
d
done
clear
View Answer play_arrow
question_answer 93) Three identical capacitors are connected together differently. For the same voltage to every combination the one that stores maximum energy is
A)
the three in series
done
clear
B)
the three in parallel
done
clear
C)
two in series and the third in parallel with it
done
clear
D)
two in parallel and the third in series with it
done
clear
View Answer play_arrow
question_answer 94)
Each resistance shown in the circuit is of\[10\,\Omega \]. The equivalent resistance between the points X and Y is
A)
\[10\,\Omega \]
done
clear
B)
\[20\,\Omega \]
done
clear
C)
\[25\,\Omega \]
done
clear
D)
\[30\,\Omega \]
done
clear
View Answer play_arrow
question_answer 95) A L-R circuit consists of an inductance of 8 mH and a resistance of \[4\,\Omega \]. The time constant of the circuit is
A)
2ms
done
clear
B)
12ms
done
clear
C)
32ms
done
clear
D)
500 s
done
clear
View Answer play_arrow
question_answer 96)
Three identical resistances A, B and C are connected as shown in the figure. The heat produced will be maximum in
A)
B
done
clear
B)
same for A, Band C
done
clear
C)
A
done
clear
D)
B and C
done
clear
View Answer play_arrow
question_answer 97) A kilowatt hour is equal to
A)
\[3.6\,\times {{10}^{6}}J\]
done
clear
B)
\[3.6\times {{10}^{4}}J\]
done
clear
C)
\[3.6\times {{10}^{3}}J\]
done
clear
D)
\[6\times {{10}^{-4}}J\]
done
clear
View Answer play_arrow
question_answer 98) Wire P and Q have the same resistance at ordinary (room) temperature. When heated, resistance of P increases and that of Q decreases. We conclude that
A)
P and Q are conductors of different materials.
done
clear
B)
P is n-type semiconductor and Q is p-type semiconductor
done
clear
C)
P is semiconductor and Q is conductor
done
clear
D)
P is conductor and Q is semiconductor
done
clear
View Answer play_arrow
question_answer 99) In the disintegration series \[_{92}^{238}U\xrightarrow{\alpha }X\xrightarrow{\text{-}}_{Z}^{A}Y\]the values of Z and A respectively will be
A)
92, 236
done
clear
B)
88, 230
done
clear
C)
90, 234
done
clear
D)
91, 234
done
clear
View Answer play_arrow
question_answer 100) The plane faces of two identical plano-convex lenses, each having focal length of 40 cm, are placed against each other to form a common convex lens. The distance from this lens at which an object must be placed to obtain a real inverted image with magnification equal to unity is
A)
80 cm
done
clear
B)
40 cm
done
clear
C)
20 cm
done
clear
D)
160 cm
done
clear
View Answer play_arrow
question_answer 101) If an organic compound has\[C=40%\], \[H=13.3%\],\[N=46.67%\] , then the empirical formula for this compound is
A)
\[C{{H}_{4}}N\]
done
clear
B)
\[{{C}_{2}}{{H}_{8}}{{N}_{2}}\]
done
clear
C)
\[C{{H}_{3}}N\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 102) \[TEL\]is
A)
petrol fuel
done
clear
B)
antiknocking compound
done
clear
C)
gasoline
done
clear
D)
insecticide
done
clear
View Answer play_arrow
question_answer 103) \[DDT\] is related to
A)
insecticide
done
clear
B)
fungicide
done
clear
C)
chlorination
done
clear
D)
florination
done
clear
View Answer play_arrow
question_answer 104) Coordination number 8 : 8 is of
A)
\[CsCl\]
done
clear
B)
\[KCl\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[ZnS\]
done
clear
View Answer play_arrow
question_answer 105) Which of the following inert gas is soluble in water?
A)
\[He\]
done
clear
B)
\[Ne\]
done
clear
C)
\[Ar\]
done
clear
D)
\[Xe\]
done
clear
View Answer play_arrow
question_answer 106) Number of peptide bonds in dipeptide is
A)
\[2\]
done
clear
B)
\[1\]
done
clear
C)
\[3\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 107) Cupellation method is used for the extraction of
A)
\[Cu\]
done
clear
B)
\[Ag\]
done
clear
C)
\[Na\]
done
clear
D)
\[Al\]
done
clear
View Answer play_arrow
question_answer 108) Blood sugar is
A)
fructose
done
clear
B)
maltose
done
clear
C)
haemoglobin
done
clear
D)
glucose
done
clear
View Answer play_arrow
question_answer 109) Lactose sugar is formed from
A)
\[d-\]galactose
done
clear
B)
\[d-\]glucose
done
clear
C)
\[d-\]galactose and d-glucose
done
clear
D)
\[d-\]glucose and d-fructose
done
clear
View Answer play_arrow
question_answer 110) The values for all the quantum numbers for 15th electron of chlorine are
A)
\[n=3,\,\,l=1,\,\,m=0,\,\,s=-1/2\]
done
clear
B)
\[n=4,\,\,l=2,\,\,m=0,\,\,s=+1/2\]
done
clear
C)
\[n=3,\,\,l=1,\,\,m=+1,\,\,s=+1/2\]
done
clear
D)
\[n=2,\,\,l=0,\,\,m=0,\,\,s=+1/2\]
done
clear
View Answer play_arrow
question_answer 111) The IUPAC name of the compound is \[C{{H}_{3}}-{{[C{{H}_{2}}]}_{5}}-\underset{\begin{align} & | \\ & C{{H}_{2}} \\ & | \\ & ^{\beta }CHC{{H}_{3}} \\ & | \\ & C{{H}_{2}} \\ & | \\ & C{{H}_{3}} \\ \end{align}}{\mathop{C}}\,H-C{{H}_{2}}-\underset{\begin{align} & | \\ & ^{\alpha }CHC{{H}_{3}} \\ & | \\ & C{{H}_{2}} \\ & | \\ & {{C}_{2}}{{H}_{5}} \\ \end{align}}{\mathop{C}}\,H-{{[C{{H}_{2}}]}_{_{3}}}-C{{H}_{3}}\]
A)
7[\[\beta \]methyl butyl], 9 butyl tridecane
done
clear
B)
3[\[\beta \]ethyl butyl], 9 ethyl tridecane
done
clear
C)
2[\[\beta \]ethyl ethenyl], 8 propyl decane
done
clear
D)
None of the above
done
clear
View Answer play_arrow
A)
\[(III)>(II)>(I)>(IV)\]
done
clear
B)
\[(II)>(III)>(I)>(IV)\]
done
clear
C)
\[(I)>(II)>(III)>(IV)\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 113) Outermost configuration for\[Z=25\], is
A)
\[4{{s}^{2}},\,\,3{{d}^{5}}\]
done
clear
B)
\[5{{s}^{2}},\,\,4{{d}^{5}}\]
done
clear
C)
\[4{{s}^{2}},\,\,3{{d}^{3}}\]
done
clear
D)
\[4{{s}^{2}},\,\,3{{d}^{1}}\]
done
clear
View Answer play_arrow
question_answer 114) The colour of \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] in acidic medium is
A)
red
done
clear
B)
green
done
clear
C)
yellow
done
clear
D)
pink
done
clear
View Answer play_arrow
question_answer 115) Baeyers reagent decolorizes which of the following?
A)
Alkane
done
clear
B)
Alkene
done
clear
C)
Alkene and alkyne both
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 116) When \[Mn{{O}_{4}}\] is fused with\[KOH\], a colored compound is formed. The product and its colour is
A)
\[KMn{{O}_{4}}\], purple green
done
clear
B)
\[KMn{{O}_{4}}\], purple
done
clear
C)
\[M{{n}_{2}}{{O}_{3}}\], brown
done
clear
D)
\[M{{n}_{2}}{{O}_{4}}\], black
done
clear
View Answer play_arrow
question_answer 117) The type of hybridization present in each carbon of benzene is
A)
\[s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[sp\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 118) Total number of isomers of \[{{C}_{4}}{{H}_{10}}O\] is
A)
7
done
clear
B)
4
done
clear
C)
3
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 119) Vitamin \[{{B}_{12}}\] contains
A)
\[Co\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Zn\]
done
clear
D)
\[Ca\]
done
clear
View Answer play_arrow
question_answer 120) Night blindness is caused due to the deficiency of
A)
\[{{B}_{12}}\]
done
clear
B)
\[A\]
done
clear
C)
\[C\]
done
clear
D)
\[D\]
done
clear
View Answer play_arrow
question_answer 121) RNA does not contain
A)
thymine
done
clear
B)
adenine
done
clear
C)
guanine
done
clear
D)
uracil
done
clear
View Answer play_arrow
question_answer 122) Reaction of dry \[HCl\] with acetone gives
A)
aldol
done
clear
B)
chloritone
done
clear
C)
isopropyl alcohol
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 123) Which of the following is incorrect for radial distribution curve?
A)
\[n\] \[l\] Node \[2\] \[0\] \[1\]
done
clear
B)
\[3\] \[0\] \[2\]
done
clear
C)
\[2\] \[1\] \[0\]
done
clear
D)
\[3\] \[2\] \[1\]
done
clear
View Answer play_arrow
question_answer 124) If \[{{H}^{+}}\] concentration of fruit juice is\[3.3\times {{10}^{-2}}\], then its \[pH\] will be
A)
\[4.8\,\,basic\]
done
clear
B)
\[4.8\,\,neutral\]
done
clear
C)
\[4.8\,\,acidic\]
done
clear
D)
\[1.6\,\,acidic\]
done
clear
View Answer play_arrow
question_answer 125) \[{{C}_{2}}{{H}_{5}}OH\xrightarrow[NaOH]{{{I}_{2}}}A\xrightarrow[KOH]{Aqueous}B\] In equation given above \[A\] and \[B\] are respectively
A)
\[CH{{I}_{3}}\]and\[HCOOK\]
done
clear
B)
\[CH{{I}_{3}}\]and\[C{{H}_{3}}COOK\]
done
clear
C)
\[C{{H}_{4}}\]and\[HCOOK\]
done
clear
D)
\[CH{{I}_{3}}\]and\[{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 126) Formula of washing soda is
A)
\[N{{a}_{2}}C{{O}_{3}}\cdot 10{{H}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
C)
\[N{{a}_{2}}S{{O}_{4}}\cdot 10{{H}_{2}}O\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 127) \[AgN{{O}_{3}}\] gives red colour with
A)
\[NaCl\]
done
clear
B)
\[NaBr\]
done
clear
C)
\[NaI\]
done
clear
D)
\[{{K}_{2}}Cr{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 128) If the value of bond order is zero, then
A)
molecule will be stable
done
clear
B)
molecule will be unstable
done
clear
C)
molecule will be in ionic state
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 129) Which is responsible for the diagonal relation of lithium with magnesium?
A)
Less ionic radii
done
clear
B)
High polarizing power
done
clear
C)
Approximately equal electronegativity and affinity
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 130)
Trioxane has the formula
A)
formaldehyde
done
clear
B)
methanol
done
clear
C)
dichloromethane
done
clear
D)
vinyl alcohol
done
clear
View Answer play_arrow
question_answer 131) Natural rubber is
A)
butadiene
done
clear
B)
isoprene
done
clear
C)
neoprene
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 132) Monomer of polythene is
A)
ethylene
done
clear
B)
propylene
done
clear
C)
vinyl chloride
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 133) Bond order of \[{{N}_{2}}\] molecule is
A)
\[3\]
done
clear
B)
\[2\]
done
clear
C)
\[1\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 134)
Which of the following is a proper match? \[\frac{{{\mathbf{r}}^{\mathbf{+}}}}{{{\mathbf{r}}^{\mathbf{-}}}}\] Shape (A)\[0.115-0.225\] (i) Triangular (B)\[0.225-0.414\] (ii) Tetrahedral (C)\[0.414-0.732\] (iii) Cubic (D)\[0.732-1\] (iv) Octahedral
A)
\[A-i,\,\,B-ii,\,\,C-iv,\,\,D-iii\]
done
clear
B)
\[A-iii,\,\,B-ii,\,\,C-iv,\,\,D-i\]
done
clear
C)
\[A-i,\,\,B-iii,\,\,C-i,\,\,D-iv\]
done
clear
D)
\[A-ii,\,\,B-iv,\,\,C-i,\,\,D-iii\]
done
clear
View Answer play_arrow
question_answer 135)
The correct order of acidity of following acids is
A)
\[>-Cl-C{{H}_{2}}COOH>HCOOH>C{{H}_{3}}COOH\]
done
clear
B)
\[F-C{{H}_{2}}COOH>Cl-C{{H}_{2}}COOH\]
done
clear
C)
\[HCOOH>C{{H}_{3}}COOH>Cl-C{{H}_{2}}COOH\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 136) \[C{{O}_{2}}\] gas obtained by the combustion of \[12\,\,mL\] butane gas is
A)
\[3\,\,mL\]
done
clear
B)
\[12\,\,mL\]
done
clear
C)
\[24\,\,mL\]
done
clear
D)
\[48\,\,mL\]
done
clear
View Answer play_arrow
question_answer 137) Calomel is
A)
\[H{{g}_{2}}C{{l}_{2}}\]
done
clear
B)
\[Hg+H{{g}_{2}}C{{l}_{2}}\]
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 138) In an adiabatic process which of the following is true?
A)
\[q=+W\]
done
clear
B)
\[q=0\]
done
clear
C)
\[\Delta E=q\]
done
clear
D)
\[p\Delta V=0\]
done
clear
View Answer play_arrow
question_answer 139) The number of paired electrons in \[Xe\] atom of \[Xe{{F}_{2}}\] is
A)
\[3\]
done
clear
B)
\[5\]
done
clear
C)
\[4\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 140) Hydrolysis of \[Xe{{F}_{6}}\] gives
A)
\[Xe{{O}_{3}}\]
done
clear
B)
\[Xe{{O}_{6}}\]
done
clear
C)
\[Xe{{O}_{2}}\]
done
clear
D)
\[Xe{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 141) Zwitter ion is
A)
dipolar ion
done
clear
B)
ion formed from amino acid
done
clear
C)
internal salt
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 142) \[IUPAC\]name of\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]is
A)
diamine dichloro platinum (II)
done
clear
B)
amine, chloro platinum (III)
done
clear
C)
chloro diamine platinum (II)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 143) Nylon obtained by the condensation of adipic acid with hexamethylene diamine
A)
nylon 6, 6
done
clear
B)
nylon 7, 6
done
clear
C)
nylon 6, 5
done
clear
D)
nylon 9, 7
done
clear
View Answer play_arrow
question_answer 144) Equation\[\log \frac{{{K}_{2}}}{{{K}_{1}}}=\frac{\Delta H}{2.303R}\left[ \frac{{{T}_{2}}-{{T}_{1}}}{{{T}_{1}}\cdot {{T}_{2}}} \right],\,\,n\]
A)
van der Waals equation
done
clear
B)
Kirchhoffs equation
done
clear
C)
Gas equation
done
clear
D)
vant Hoff equation
done
clear
View Answer play_arrow
question_answer 145) Which of the following is a colligative property?
A)
Surface tension
done
clear
B)
Viscosity
done
clear
C)
Osmotic pressure
done
clear
D)
Vapour pressure
done
clear
View Answer play_arrow
question_answer 146) Number of \[\alpha \] and \[\beta \] emitted in the reaction \[_{92}{{U}^{238}}{{\xrightarrow{{}}}_{82}}P{{b}^{206}}\]are
A)
\[6\alpha \]and\[8\beta \]
done
clear
B)
\[8\alpha \]and\[6\beta \]
done
clear
C)
\[6\alpha \]and\[4\beta \]
done
clear
D)
\[4\alpha \]and\[6\beta \]
done
clear
View Answer play_arrow
question_answer 147) A chemical reaction is catalyzed by \[X\] therefore,\[X\]
A)
increases the activation energy
done
clear
B)
does not affect the equilibrium constant of the reaction
done
clear
C)
decreases the enthalpy of the reaction
done
clear
D)
decreases the velocity constant of the reaction
done
clear
View Answer play_arrow
question_answer 148) The half-life of a radioactive isotope is\[3\,\,h\]. If the initial mass of the isotope were\[256\,\,g\], the mass of it remaining undecayed after \[18\,\,h\] would be
A)
\[8.0\,\,g\]
done
clear
B)
\[4.0\,\,g\]
done
clear
C)
\[12.0\,\,g\]
done
clear
D)
\[16.0\,\,g\]
done
clear
View Answer play_arrow
question_answer 149) According to \[MOT\] which of the following is correct for potassium hexa cyano ferrate (III)?
A)
It is a octahedral complex
done
clear
B)
\[{{t}_{2g}}\] orbital contains the e of metal
done
clear
C)
For iron, overlapping between empty orbitals and ligand orbitals takes place
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 150) Radioactive hydrogen is
A)
tritium
done
clear
B)
deuterium
done
clear
C)
ortho hydrogen
done
clear
D)
para hydrogen
done
clear
View Answer play_arrow
question_answer 151) Percentage of oxygen in phenol is
A)
\[17.02\]
done
clear
B)
\[15.2\]
done
clear
C)
\[16\]
done
clear
D)
\[15\]
done
clear
View Answer play_arrow
question_answer 152) Which of the following differs in\[EAN\]?
A)
\[[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
done
clear
B)
\[{{K}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
C)
\[{{K}_{2}}[ZnC{{l}_{4}}]\]
done
clear
D)
\[{{K}_{2}}[Hg{{I}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 153) Which of the following complex neutralizes three molecules of\[AgN{{O}_{3}}\]?
A)
\[[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]
done
clear
View Answer play_arrow
question_answer 154) \[Zn|\underset{C=1}{\mathop{Z{{n}^{2+}}}}\,||\underset{C=1}{\mathop{C{{u}^{2+}}}}\,|Cu\] If the standard reduction potential of zinc electrode and copper half-cell is \[-0.76\,\,V\] and \[0.34\,\,V\] respectively then the emf will be
A)
\[1.1\,\,V\]
done
clear
B)
\[1.4\,\,V\]
done
clear
C)
\[1.34\,\,V\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 155) Which of the following does not show optical isomerization?
A)
\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
B)
\[{{[Co{{(ox)}_{3}}]}^{3-}}\]
done
clear
C)
\[{{[Co{{(en)}_{3}}]}^{3+}}\]
done
clear
D)
\[{{[Cr{{(diph)}_{3}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 156) Solubility product of pure \[PbC{{l}_{2}}\] will be
A)
\[{{K}_{sp}}={{S}^{2}}\]
done
clear
B)
\[{{K}_{sp}}=4{{S}^{3}}\]
done
clear
C)
\[{{K}_{sp}}=108\,\,{{S}^{5}}\]
done
clear
D)
\[{{K}_{sp}}=S\]
done
clear
View Answer play_arrow
question_answer 157) The expression for velocity constant for the second order reaction is
A)
\[k=\frac{2.303}{t}{{\log }_{10}}\frac{a}{a-x}\]
done
clear
B)
\[k=\frac{1}{t}\frac{x}{a[a-x]}\]
done
clear
C)
\[k=\frac{1}{t}\frac{{{x}^{2}}}{{{a}^{2}}{{[a-x]}^{2}}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 158) The unit of rate constant for second order reaction is
A)
\[{{s}^{-1}}\]
done
clear
B)
\[mol/{{L}^{-1}}\]
done
clear
C)
\[L\,\,mo{{l}^{-1}}{{s}^{-1}}\]
done
clear
D)
\[{{L}^{2}}mo{{l}^{-2}}s\]
done
clear
View Answer play_arrow
question_answer 159) Which of the following, on heating with ammonia gives urotropin?
A)
Formaldehyde
done
clear
B)
Acetaldehyde
done
clear
C)
Acetone
done
clear
D)
Benzaldehyde
done
clear
View Answer play_arrow
question_answer 160) Which of the following statement is correct?
A)
Basic nature increases on increasing pH
done
clear
B)
Basic nature decreases on increasing pH
done
clear
C)
Acidic nature increases on increasing pH
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 161) For the redox reaction \[Zn(s)+C{{u}^{2+}}(0.1\,\,M)\xrightarrow{{}}Z{{n}^{2+}}(1M)+Cu(s)\] taking place in a cell, \[E_{cell}^{\text{o}}\] is\[1.10\,\,V\]. \[{{E}_{cell}}\] for the cell will be\[\left( 2.3303\frac{RT}{F}=0.0591 \right)\]
A)
\[2.14\,\,V\]
done
clear
B)
\[1.80\,\,V\]
done
clear
C)
\[0.82\,\,V\]
done
clear
D)
\[1.07\,\,V\]
done
clear
View Answer play_arrow
question_answer 162) \[{{C}_{6}}{{H}_{6}}+C{{l}_{2}}\xrightarrow{Sunlight}\]product
A)
\[BHC\]
done
clear
B)
chloro benzene
done
clear
C)
dichloro benzene
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 163) The required condition for an ideal solution is
A)
\[\Delta {{H}_{mix}}=0\]
done
clear
B)
\[\Delta {{V}_{mix}}\ne 0\]
done
clear
C)
\[{{p}_{A}}\ne p_{A}^{o}{{X}_{A}}\]
done
clear
D)
\[\Delta {{H}_{mix}}>1\]
done
clear
View Answer play_arrow
question_answer 164) Interionic distance for \[CsCl\] crystal is
A)
\[a\]
done
clear
B)
\[a/2\]
done
clear
C)
\[\frac{\sqrt{3}}{4}a\]
done
clear
D)
\[\frac{2a}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 165) How many space lattices are obtained from the different crystal systems?
A)
7
done
clear
B)
14
done
clear
C)
32
done
clear
D)
230
done
clear
View Answer play_arrow
question_answer 166) Which of the following occurs at anode?
A)
Reduction
done
clear
B)
Oxidation
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 167) Parkes process is used for the extraction of
A)
iron
done
clear
B)
sodium
done
clear
C)
silver
done
clear
D)
zinc
done
clear
View Answer play_arrow
question_answer 168) The common method of extraction of metals from oxide ores is
A)
reduction with carbon
done
clear
B)
reduction with hydrogen
done
clear
C)
reduction with aluminium
done
clear
D)
electrolytic method
done
clear
View Answer play_arrow
question_answer 169) The transition metals mostly are
A)
diamagnetic
done
clear
B)
paramagnetic
done
clear
C)
neither diamagnetic nor paramagnetic
done
clear
D)
both diamagnetic and paramagnetic
done
clear
View Answer play_arrow
question_answer 170) Which of the following is an electron deficient compound?
A)
\[{{B}_{2}}{{H}_{6}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
D)
\[CC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 171) Aniline reacts with chloroform in the presence of \[KOH\] to give
A)
phenol
done
clear
B)
chlorobenzene
done
clear
C)
phenyl cyanide
done
clear
D)
phenyl isocyanide
done
clear
View Answer play_arrow
question_answer 172) Which one of the following is conjugate acid of water in the reaction? \[{{H}_{2}}S{{O}_{4}}+{{H}_{2}}O{{H}_{3}}{{O}^{+}}+HSO_{4}^{-}\]
A)
\[{{H}_{2}}O\]
done
clear
B)
\[{{H}_{3}}{{O}^{+}}\]
done
clear
C)
\[SO_{4}^{2-}\]
done
clear
D)
\[HSO_{4}^{-}\]
done
clear
View Answer play_arrow
question_answer 173) The product of \[{{H}^{+}}\] and \[O{{H}^{-}}\] of water will be
A)
\[{{K}_{w}}={{10}^{-12}}\]
done
clear
B)
\[{{K}_{w}}={{10}^{-14}}\]
done
clear
C)
\[{{K}_{w}}={{10}^{-11}}\]
done
clear
D)
\[{{K}_{w}}={{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 174) When methyl cyanide is hydrolyzed in presence of alkali, the product is
A)
acetamide
done
clear
B)
methane
done
clear
C)
\[C{{O}_{2}}+{{H}_{2}}O\]
done
clear
D)
acetic acid
done
clear
View Answer play_arrow
question_answer 175) For\[NaCl\], the\[{{K}_{sp}}=36\,\,mo{{l}^{2}}{{L}^{-2}}\], the molar concentration of it will be
A)
\[\frac{1}{36}M\]
done
clear
B)
\[\frac{1}{16}M\]
done
clear
C)
\[36\,\,M\]
done
clear
D)
\[6\,\,M\]
done
clear
View Answer play_arrow
question_answer 176) Four solutions \[A,\,\,B,\,\,C,\,\,D\] has glucose\[0.5\,\,M\], \[NaCl\,\,0.1\,\,M\], \[BaC{{l}_{2}}\,\,0.5\,\,M\] and\[MgC{{l}_{2}}\,\,0.1\,\,M\], then which of the following will have highest osmotic pressure?
A)
Glucose
done
clear
B)
\[BaC{{l}_{2}}\]
done
clear
C)
\[MgC{{l}_{2}}\]
done
clear
D)
\[NaCl\]
done
clear
View Answer play_arrow
question_answer 177) The highest boiling point is of
A)
\[FeC{{l}_{2}}\]
done
clear
B)
\[FeC{{l}_{3}}\]
done
clear
C)
\[A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\]
done
clear
D)
\[AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 178) For an ionic crystal of the general formula \[M\] and coordination number is\[6\], the value of radius ratio will be
A)
greater than 0.73
done
clear
B)
in between 0.73 and 0.41
done
clear
C)
in between 0.41 and 0.22
done
clear
D)
less than 0.22
done
clear
View Answer play_arrow
question_answer 179) Which of the following can be used to convert \[_{7}^{14}N\]into\[_{8}^{17}O\]?
A)
Deutron
done
clear
B)
Proton
done
clear
C)
\[\alpha \]-particle
done
clear
D)
Neutron
done
clear
View Answer play_arrow
question_answer 180) \[{{N}_{2}}{{O}_{4}}2N{{O}_{2}}-Q\] The unit of \[{{K}_{p}}\] for the given reaction is
A)
atmosphere
done
clear
B)
atomsphere2
done
clear
C)
atmosphere-1
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 181) Number of ions for the complex\[[Co{{(N{{H}_{3}})}_{4}}Cl]C{{l}_{2}}\] shown by the conductivity measurement, is
A)
2
done
clear
B)
4
done
clear
C)
3
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 182) Rusting on iron needs
A)
dry air
done
clear
B)
air and water
done
clear
C)
distilled water and carbon dioxide
done
clear
D)
oxygen and carbon dioxide
done
clear
View Answer play_arrow
question_answer 183) First ionization potential is highest for
A)
\[Na\]
done
clear
B)
\[Mg\]
done
clear
C)
\[Al\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 184) Which compound is not a lipid?
A)
Lecithin
done
clear
B)
Lysine
done
clear
C)
Cerebroside
done
clear
D)
Cephalin
done
clear
View Answer play_arrow
question_answer 185) Which one of the following notations shows product incorrectly?
A)
\[_{96}^{242}Cm(\alpha ,\,\,2m)_{97}^{243}Bk\]
done
clear
B)
\[_{5}^{10}B(\alpha ,\,\,n)_{7}^{13}N\]
done
clear
C)
\[_{7}^{14}N(n,\,\,p)_{6}^{14}C\]
done
clear
D)
\[_{14}^{28}Si(d,\,\,n)_{15}^{29}P\]
done
clear
View Answer play_arrow
question_answer 186) In the equilibrium reaction\[2HI(g){{H}_{2}}+{{I}_{3}}\]which of the following expressions is true?
A)
\[{{K}_{p}}={{K}_{c}}\]
done
clear
B)
\[{{K}_{c}}=2{{K}_{p}}\]
done
clear
C)
\[{{K}_{p}}>{{K}_{c}}\]
done
clear
D)
\[{{K}_{c}}={{K}_{p}}{{(RT)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 187) For a reaction of the type \[A+B\to \] products, it is observed that doubling the concentration of \[A\] causes the reaction rate to be four times as great, but doubling the amount of \[B\] does not affect the rate. The rate equation is
A)
\[Rate=k[A][B]\]
done
clear
B)
\[Rate=k{{[A]}^{2}}\]
done
clear
C)
\[Rate=k{{[A]}^{2}}[B]\]
done
clear
D)
\[Rate=k{{[A]}^{2}}[B]\]
done
clear
View Answer play_arrow
question_answer 188) In the following, the element with the highest electropositivity is
A)
copper
done
clear
B)
cesium
done
clear
C)
barium
done
clear
D)
chromium
done
clear
View Answer play_arrow
question_answer 189) The reaction \[C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}\xrightarrow[{{250}^{o}}-{{300}^{o}}C]{Ni}C{{H}_{3}}-C{{H}_{3}}\] is called
A)
Wurtz reaction
done
clear
B)
Kolbes reaction
done
clear
C)
Sabatier-Senderens reaction
done
clear
D)
Carbylamine reaction
done
clear
View Answer play_arrow
question_answer 190) Unit of first order rate constant is
A)
\[mol\,\,L\,\,s\]
done
clear
B)
\[mo{{l}^{-1}}{{L}^{-1}}{{s}^{-1}}\]
done
clear
C)
\[mol\,\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
D)
\[{{s}^{-1}},\,\,{{\min }^{-1}}etc\].
done
clear
View Answer play_arrow
question_answer 191) Which of the following describes the shape of orbital?
A)
Principal quantum number
done
clear
B)
Azimuthal quantum number
done
clear
C)
Magnetic quantum number
done
clear
D)
Spin quantum number
done
clear
View Answer play_arrow
question_answer 192) The formula of a phosphate of a metal\[M\], is\[MP{{O}_{4}}\]. The formula of its nitrate will be
A)
\[MN{{O}_{3}}\]
done
clear
B)
\[M{{(N{{O}_{3}})}_{2}}\]
done
clear
C)
\[M{{(N{{O}_{3}})}_{3}}\]
done
clear
D)
\[{{M}_{2}}{{(N{{O}_{3}})}_{2}}\]
done
clear
View Answer play_arrow
question_answer 193) The \[d-\]block element consists mostly of
A)
monovalent metals
done
clear
B)
all non-metals
done
clear
C)
elements which generally form stoichiometric metal oxide
done
clear
D)
many metals with catalytic properties
done
clear
View Answer play_arrow
question_answer 194) A mixture containing \[C{{u}^{2+}}\] and \[N{{i}^{2+}}\] can be separated for identification by
A)
passing \[{{H}_{2}}S\] in acid medium
done
clear
B)
passing \[{{H}_{2}}S\] in alkaline medium
done
clear
C)
passing \[{{H}_{2}}S\] in neutral medium
done
clear
D)
passing \[{{H}_{2}}S\] in dry mixture
done
clear
View Answer play_arrow
question_answer 195) Strongest Bronsted base among the following anions is
A)
\[ClO\]
done
clear
B)
\[Cl{{O}_{2}}\]
done
clear
C)
\[ClO_{3}^{-}\]
done
clear
D)
\[ClO_{4}^{-}\]
done
clear
View Answer play_arrow
question_answer 196) Which one of the following is a good conductor of electricity?
A)
Diamond
done
clear
B)
Graphite
done
clear
C)
Silicon
done
clear
D)
Amorphous carbon
done
clear
View Answer play_arrow
question_answer 197) Which one of the following is an example of non-typical transition elements?
A)
\[Li,\,\,K,\,\,Na\]
done
clear
B)
\[Be,\,\,Al,\,\,Pb\]
done
clear
C)
\[Zn,\,\,Cd,\,\,Hg\]
done
clear
D)
\[Ba,\,\,Ca,\,\,Sr\]
done
clear
View Answer play_arrow
question_answer 198) Which of the following has zero dipole moment?
A)
\[ClF\]
done
clear
B)
\[PC{{l}_{3}}\]
done
clear
C)
\[Si{{F}_{4}}\]
done
clear
D)
\[CFC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 199) In galvanic cell, the salt bridge is used to
A)
complete the circuit
done
clear
B)
reduce the electric resistance in the cell
done
clear
C)
separate cathode from anode
done
clear
D)
carry salts for the chemical reaction
done
clear
View Answer play_arrow
question_answer 200) Reduction of methyl isocyanide gives
A)
ethylamine
done
clear
B)
methylamine
done
clear
C)
dimethylamine
done
clear
D)
trimethylamine
done
clear
View Answer play_arrow
question_answer 201) Perianth is represented by
A)
glumes
done
clear
B)
lemma
done
clear
C)
lodicules
done
clear
D)
palea
done
clear
View Answer play_arrow
question_answer 202) Table sugar is consist of
A)
lactose
done
clear
B)
sucrose
done
clear
C)
maltose
done
clear
D)
glucose
done
clear
View Answer play_arrow
question_answer 203) The terminator gene technology causes
A)
failure of seed setting after one generation
done
clear
B)
breakage of seed dormancy
done
clear
C)
early flowering in plants
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 204) The term totipotency refers to
A)
the capability of organism to regenerate its lost parts
done
clear
B)
capability of somatic cells to produce complete organism
done
clear
C)
the introduction of foreign gene in a cells DNA
done
clear
D)
the technique of growing immature embryos
done
clear
View Answer play_arrow
question_answer 205) Work of Beadle and Tatum on Neurospora rassa proved that
A)
Replication of DNA is semi-conservative
done
clear
B)
viruses have genetic material
done
clear
C)
every gene is responsible for specific enzymes
done
clear
D)
plant cells are to tipotent
done
clear
View Answer play_arrow
question_answer 206) Polyploidy means occurrence of
A)
haploid sets of chromosomes
done
clear
B)
diploid sets of chromosomes
done
clear
C)
more than diploid sets of chromosomes
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 207) The deteriorative processes in plants, that naturally terminate their functional life, are collectively called
A)
wilting
done
clear
B)
abscission
done
clear
C)
plasmolysis
done
clear
D)
senescence
done
clear
View Answer play_arrow
question_answer 208) Which pigment involves in photoperiodic change in plants?
A)
Phytochrome
done
clear
B)
Cytochrome
done
clear
C)
Chlorophyll
done
clear
D)
Anthocyanin
done
clear
View Answer play_arrow
question_answer 209) Linnaen system of plant classification is based on
A)
morphological and anatomical characters
done
clear
B)
evolutionary trends
done
clear
C)
floral characters
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 210) Succession on secondary base area is
A)
primosere
done
clear
B)
subsere
done
clear
C)
xerosere
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 211) An unrestricted reproductive capacity is called
A)
birth rate
done
clear
B)
biotic potential
done
clear
C)
carrying capacity
done
clear
D)
fertility
done
clear
View Answer play_arrow
question_answer 212) Ubisch bodies are secreted by
A)
tapetum
done
clear
B)
exine
done
clear
C)
microspore mother cells
done
clear
D)
endothecium
done
clear
View Answer play_arrow
question_answer 213) Alginic acid is found in the cell wall of
A)
Gigartina
done
clear
B)
Laminaria
done
clear
C)
Gelidium
done
clear
D)
Scytonema
done
clear
View Answer play_arrow
question_answer 214) Lady finger (bhindi) belongs to
A)
Malvaceae
done
clear
B)
Cruciferae
done
clear
C)
Solanaceae
done
clear
D)
Liliaceae
done
clear
View Answer play_arrow
question_answer 215) P-proteins are associated with
A)
sieve tube elements
done
clear
B)
xylem parenchyma
done
clear
C)
trichomes
done
clear
D)
tracheids and vessels
done
clear
View Answer play_arrow
question_answer 216) Potato is a modification of
A)
stem
done
clear
B)
rhizome
done
clear
C)
root
done
clear
D)
leaf
done
clear
View Answer play_arrow
question_answer 217) Antherozoids of Dryopteris are
A)
multiciliated and coiled
done
clear
B)
multiciliated and sickle-shaped
done
clear
C)
biciliated and coiled
done
clear
D)
biciliated and sickle-shaped
done
clear
View Answer play_arrow
question_answer 218) Ginger multiplies vegetatively by
A)
bud
done
clear
B)
tuber
done
clear
C)
stem
done
clear
D)
rhizome
done
clear
View Answer play_arrow
question_answer 219) In Cycas stem, open vascular bundle is characterized by
A)
phloem being sandwitched between xylem
done
clear
B)
cambium present in between xylem and phloem
done
clear
C)
xylem being sandwitched between phloem
done
clear
D)
xylem and phloem occurring on different radii
done
clear
View Answer play_arrow
question_answer 220) From which part of coconut coir is obtained?
A)
Pericarp
done
clear
B)
Mesocarp
done
clear
C)
Epicarp
done
clear
D)
Endocarp
done
clear
View Answer play_arrow
question_answer 221) Both heterospory and circinate plyxis occur in
A)
Dryopteris
done
clear
B)
Finns
done
clear
C)
Cycas
done
clear
D)
Funaria
done
clear
View Answer play_arrow
question_answer 222) In Funaria, the stomata are found on
A)
foot
done
clear
B)
seta
done
clear
C)
capsule
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 223) Tracheophyta consists of
A)
bryophytes only
done
clear
B)
pteridophytes only
done
clear
C)
gymnosperms and angiosperms
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
question_answer 224) Green-house effect is mainly caused by
A)
CFCs
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[CO\]
done
clear
View Answer play_arrow
question_answer 225) Nucellar polyembryony occurs in
A)
Corchorus
done
clear
B)
Cirrus
done
clear
C)
Carthamus
done
clear
D)
Zea mays
done
clear
View Answer play_arrow
question_answer 226) Male gametophyte of angiosperms is reduced o
A)
one cell
done
clear
B)
two cells
done
clear
C)
three cells
done
clear
D)
four cells
done
clear
View Answer play_arrow
question_answer 227) In \[{{C}_{3}}\] plants, the first stable product of photosynthesis during dark reaction is
A)
PGAL
done
clear
B)
RuBP
done
clear
C)
PGA
done
clear
D)
OAA
done
clear
View Answer play_arrow
question_answer 228) During the formation of embryo sac, the functional megaspore undergoes
A)
two mitotic divisions
done
clear
B)
two meiotic divisions
done
clear
C)
three meiotic divisions
done
clear
D)
three mitotic divisions
done
clear
View Answer play_arrow
question_answer 229) The first \[C{{O}_{4}}\] acceptor in \[{{C}_{4}}\] cycle is
A)
RuBP
done
clear
B)
PEP
done
clear
C)
PGA
done
clear
D)
OAA
done
clear
View Answer play_arrow
question_answer 230) The water available to plants for absorption is
A)
gravitational water
done
clear
B)
hygroscopic water
done
clear
C)
capillary water
done
clear
D)
chemically bound water
done
clear
View Answer play_arrow
question_answer 231) Cell wall of fungi is made up of
A)
fungal cellulose
done
clear
B)
hemicellulose
done
clear
C)
fungal chitin
done
clear
D)
Both (a) and (c)
done
clear
View Answer play_arrow
question_answer 232) The plant ash indicates
A)
organic matter of plant
done
clear
B)
mineral salts absorbed by plants
done
clear
C)
both mineral salts and organic matter
done
clear
D)
silica absorbed by plants
done
clear
View Answer play_arrow
question_answer 233) During cell cycle, RNA and non-histone proteins are synthesized in
A)
S-phase
done
clear
B)
\[{{G}_{\text{o}}}\]-phase
done
clear
C)
\[{{G}_{2}}\]-phase
done
clear
D)
M-phase
done
clear
View Answer play_arrow
question_answer 234) Which one of the following is the terminal electron acceptor?
A)
Molecular \[C{{O}_{2}}\]
done
clear
B)
Molecular \[{{O}_{2}}\]
done
clear
C)
Molecular \[{{H}_{2}}\]
done
clear
D)
\[NADP{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 235) Bract is a modified
A)
petal
done
clear
B)
sepal
done
clear
C)
leaf
done
clear
D)
involucres
done
clear
View Answer play_arrow
question_answer 236) Hormone replacing the requirement of vernalization is
A)
ethylene
done
clear
B)
auxin
done
clear
C)
gibberellins
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 237) Thigmotropism is best seen in
A)
tendrils
done
clear
B)
leaf apex
done
clear
C)
root apex
done
clear
D)
stem apex
done
clear
View Answer play_arrow
question_answer 238) Transpiration is measured by
A)
potometre
done
clear
B)
porometre
done
clear
C)
auxanometre
done
clear
D)
respirometre
done
clear
View Answer play_arrow
question_answer 239) The function of polymerase chain reaction is
A)
transduction
done
clear
B)
DNA amplification
done
clear
C)
translation
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 240) Mutation is more common when it is present in
A)
recessive condition
done
clear
B)
dominant condition
done
clear
C)
constant in population
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 241) The most common type of ovule in angiosperms is
A)
amphitropous
done
clear
B)
atropous
done
clear
C)
anatropous
done
clear
D)
circinotropous
done
clear
View Answer play_arrow
question_answer 242) When two hybrids rrTt and Rrtt are crossed, the phenotype ratio of offspring would be
A)
3 : 1
done
clear
B)
9 : 3 : 3 : 1
done
clear
C)
1 : 1
done
clear
D)
1 : 1 : 1 : 1
done
clear
View Answer play_arrow
question_answer 243) One of the most resistant known biological material is
A)
lignin
done
clear
B)
hemicellulose
done
clear
C)
sporopollenin
done
clear
D)
lignocelluloses
done
clear
View Answer play_arrow
question_answer 244) Energy enters the ecosystem through
A)
herbivore
done
clear
B)
carnivore
done
clear
C)
producer
done
clear
D)
decomposer
done
clear
View Answer play_arrow
question_answer 245) In soil profile, humus is present in
A)
horizon-O
done
clear
B)
horizon-A
done
clear
C)
horizon-B
done
clear
D)
horizon-C
done
clear
View Answer play_arrow
question_answer 246) The smallest angiospermic flower is
A)
Wolffia
done
clear
B)
Ranunculus
done
clear
C)
Rafflesia
done
clear
D)
SteHana
done
clear
View Answer play_arrow
question_answer 247) The pyramid of energy is always
A)
opaque
done
clear
B)
horizontal
done
clear
C)
upright
done
clear
D)
inverted
done
clear
View Answer play_arrow
question_answer 248) The transition zone between the two vegetations of ecosystem is called
A)
ecotone
done
clear
B)
ecocline
done
clear
C)
ecosystem
done
clear
D)
ecesis
done
clear
View Answer play_arrow
question_answer 249) Protein in silk thread is
A)
fibroin
done
clear
B)
keratin
done
clear
C)
albumin
done
clear
D)
globulin
done
clear
View Answer play_arrow
question_answer 250) Thermoregulatory centre of human body is associated with
A)
cerebrum
done
clear
B)
cerebellum
done
clear
C)
hypothalamus
done
clear
D)
medulla oblongata
done
clear
View Answer play_arrow
question_answer 251) Body cavity of adult Ascans is
A)
haemocoel
done
clear
B)
amphicoel
done
clear
C)
pseudocoel
done
clear
D)
schizocoel
done
clear
View Answer play_arrow
question_answer 252) Collar cells are characteristic of
A)
earthworm
done
clear
B)
roundworms
done
clear
C)
coelenterata
done
clear
D)
sponges
done
clear
View Answer play_arrow
question_answer 253) In honey bee, the drones are
A)
sterile male
done
clear
B)
fertile male
done
clear
C)
fertile female
done
clear
D)
sterile female
done
clear
View Answer play_arrow
question_answer 254) Crypts of Leiberkuhn are involved in
A)
secretion of succus entericus
done
clear
B)
secretion of rennin
done
clear
C)
secretion of ptyalin
done
clear
D)
digestion of food
done
clear
View Answer play_arrow
question_answer 255) Plasmids are found in
A)
virus
done
clear
B)
bacteria
done
clear
C)
fungi
done
clear
D)
viroid
done
clear
View Answer play_arrow
question_answer 256) Oxygen dissociation curve is
A)
sigmoid
done
clear
B)
parabolic
done
clear
C)
hyperbolic
done
clear
D)
straight line
done
clear
View Answer play_arrow
question_answer 257) Blood leaving the liver and going towards heart is rich in
A)
bile
done
clear
B)
urea
done
clear
C)
ammonia
done
clear
D)
oxygen
done
clear
View Answer play_arrow
question_answer 258) Membrane that covers the vacuole in a plant cell is called
A)
tonoplast
done
clear
B)
tonoplasm
done
clear
C)
jacket
done
clear
D)
cell membrane
done
clear
View Answer play_arrow
question_answer 259) In earthworm, gizzard is found, in which of the following segments?
A)
9th segment
done
clear
B)
18th segment
done
clear
C)
13th segment
done
clear
D)
16th segment
done
clear
View Answer play_arrow
question_answer 260) The infective stage of Entamoeba histolytica is
A)
trophozoite stage
done
clear
B)
binucleated cyst stage
done
clear
C)
tetranucleated cyst stage
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 261) The initiation codon in eukaryotes is
A)
AUG
done
clear
B)
UGA
done
clear
C)
UAG
done
clear
D)
UAA
done
clear
View Answer play_arrow
question_answer 262) Pasteurization temperature is
A)
\[72{}^\circ C\] for 20 minutes
done
clear
B)
\[63{}^\circ C\] for 15 seconds
done
clear
C)
\[70{}^\circ C\] for 15 seconds
done
clear
D)
\[65{}^\circ C\] for 30 minutes
done
clear
View Answer play_arrow
question_answer 263) The number of heart chambers found in cockroach is
A)
4
done
clear
B)
7
done
clear
C)
5
done
clear
D)
13
done
clear
View Answer play_arrow
question_answer 264) The ratio of methane, ammonia and hydrogen in Stanley Millers experiment was
A)
3 : 1 : 2
done
clear
B)
2 : 1 : 2
done
clear
C)
1 : 2 : 1
done
clear
D)
5 : 4 : 1
done
clear
View Answer play_arrow
question_answer 265) Convergent evolution is shown by
A)
homologous organ
done
clear
B)
analogous organ
done
clear
C)
vestigial organ
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 266) Teichoic acid is present in
A)
cell wall of Gram positive bacteria
done
clear
B)
cell wall of Gram negative bacteria
done
clear
C)
capsid of virus
done
clear
D)
protoplasm of mycoplasma
done
clear
View Answer play_arrow
question_answer 267) % sign is used for
A)
actinomorphic flower
done
clear
B)
zygomorphic flower
done
clear
C)
incomplete flower
done
clear
D)
epigynous flower
done
clear
View Answer play_arrow
question_answer 268) Crossing over occurs
A)
single strand stage
done
clear
B)
two strand stage
done
clear
C)
four strand stage
done
clear
D)
eight strand stage
done
clear
View Answer play_arrow
question_answer 269) Ontogeny repeats phylogeny is the statement of which of the following theories?
A)
Mutation theory
done
clear
B)
Inheritance theory
done
clear
C)
Recapitulation theory
done
clear
D)
Natural selection theory
done
clear
View Answer play_arrow
question_answer 270) Darwin proposed the theory of
A)
inheritance of acquired characters
done
clear
B)
natural selection
done
clear
C)
recapitulation
done
clear
D)
continuity of germplasm
done
clear
View Answer play_arrow
question_answer 271) Which of the following is not Darwins conclusion?
A)
Survival of the fittest
done
clear
B)
Struggle for existence
done
clear
C)
Inheritance of acquired characters
done
clear
D)
Origin of species by natural selection
done
clear
View Answer play_arrow
question_answer 272) Nuclear membrane is continuous with
A)
rough endoplasmic reticulum
done
clear
B)
smooth endoplasmic reticulum
done
clear
C)
cell membrane
done
clear
D)
Golgi bodies
done
clear
View Answer play_arrow
question_answer 273) Cosmid is
A)
extragenetic material in mycoplasma
done
clear
B)
circular DNA in bacteria
done
clear
C)
extra DNA in bacteria
done
clear
D)
fragment of DNA inserted in bacteria for forming copies
done
clear
View Answer play_arrow
question_answer 274) XO chromosomal abnormality in humans causes
A)
Turners syndrome
done
clear
B)
Downs syndrome
done
clear
C)
Darwins syndrome
done
clear
D)
Klinefelters syndrome
done
clear
View Answer play_arrow
question_answer 275) Fertilization of ovum takes place in rabbit, man and other placental mammals in
A)
ovary
done
clear
B)
fallopian tube
done
clear
C)
cervix
done
clear
D)
uterus
done
clear
View Answer play_arrow
question_answer 276) At what stage in test tube babies, the zygote is implanted in human female?
A)
32-celled stage
done
clear
B)
64-celled stage
done
clear
C)
100-celled stage
done
clear
D)
164-celled stage
done
clear
View Answer play_arrow
question_answer 277) Pentoses and hexoses are common
A)
monosaccharides
done
clear
B)
disaccharides
done
clear
C)
polysaccharides
done
clear
D)
oligosaccharides
done
clear
View Answer play_arrow
question_answer 278) Pheromone is
A)
a product of endocrine gland
done
clear
B)
used for animal communication
done
clear
C)
messenger RNA
done
clear
D)
always protein
done
clear
View Answer play_arrow
question_answer 279) Secretion is under control of neurosecretory nerve axons in
A)
pineal gland
done
clear
B)
adrenal cortex
done
clear
C)
anterior pituitary
done
clear
D)
posterior pituitary
done
clear
View Answer play_arrow
question_answer 280) If an isolated strain of DNA is kept at \[82-90{}^\circ C\] then
A)
it changes into RNA
done
clear
B)
it breaks into two fragments
done
clear
C)
it breaks into many fragments
done
clear
D)
it uncoils and the two strands separate
done
clear
View Answer play_arrow
question_answer 281) The smallest endocrine gland is
A)
thyroid
done
clear
B)
parathyroid
done
clear
C)
pituitary
done
clear
D)
adrenal
done
clear
View Answer play_arrow
question_answer 282) Ban body in mammals represents
A)
all the heterochromatin in female cells
done
clear
B)
one of the two X-chromosomes in somatic cells of females
done
clear
C)
all the heterochromatin in male and female cells
done
clear
D)
the Y-chromosome in somatic cells of male
done
clear
View Answer play_arrow
question_answer 283) Gland responsible for calcium metabolism is
A)
thymus
done
clear
B)
thyroid
done
clear
C)
parathyroid
done
clear
D)
adrenal
done
clear
View Answer play_arrow
question_answer 284) Which of the following is not a case of epimorphosis?
A)
Formation of sperms from small clumps of cells
done
clear
B)
Regeneration of tail in a lizard
done
clear
C)
Replacement of severed arm in starfish
done
clear
D)
Replacement of limb in salamander
done
clear
View Answer play_arrow
question_answer 285) The daughter born to haemophilic father and normal mother could be
A)
normal
done
clear
B)
carrier
done
clear
C)
haemophilic
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 286) Removal or absence of thymus in early life shall bring about
A)
lack of lymphocytes
done
clear
B)
lack of antibodies
done
clear
C)
lack of lymph nodes
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 287) Bone marrow is made up of
A)
muscular fibre and fatty tissue
done
clear
B)
fatty tissue and areolar tissue
done
clear
C)
fatty tissue and cartilage
done
clear
D)
fatty tissue, areolar tissue and blood vessel
done
clear
View Answer play_arrow
question_answer 288) Mast cells secrete
A)
serotonin
done
clear
B)
heparin
done
clear
C)
histamine
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 289) Which one is component of ornithine cycle?
A)
Ornithine, citrulline and fumaric acid
done
clear
B)
Ornithine, citrulline and arginine
done
clear
C)
Ornithine, citrulline and alanine
done
clear
D)
Amino acids are not used
done
clear
View Answer play_arrow
question_answer 290) Bidders canal is present in
A)
male rabbit
done
clear
B)
male frog
done
clear
C)
female frog
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
question_answer 291) Zygomatic arch of rabbit is formed of
A)
maxilla, periotic and jugal
done
clear
B)
periotic, jugal and palatine
done
clear
C)
maxilla, squamosal and jugal
done
clear
D)
maxilla, premaxilla and squamosal
done
clear
View Answer play_arrow
question_answer 292) Role of spleen in mammal is
A)
to control blood pressure
done
clear
B)
to assist liver
done
clear
C)
to act as haemopoietic tissue
done
clear
D)
to assist kidneys
done
clear
View Answer play_arrow
question_answer 293) Excretory product of spider is
A)
uric acid
done
clear
B)
ammonia
done
clear
C)
guanine
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 294) Green glands present in some arthropods help in
A)
respiration
done
clear
B)
excretion
done
clear
C)
digestion
done
clear
D)
reproduction
done
clear
View Answer play_arrow
question_answer 295) Sensation of stomach pain is due to
A)
interoceptors
done
clear
B)
exteroceptors
done
clear
C)
proprioceptors
done
clear
D)
teloceptors
done
clear
View Answer play_arrow
question_answer 296) Right lung of rabbit is divided into
A)
four lobes
done
clear
B)
two lobes
done
clear
C)
six lobes
done
clear
D)
eight lobes
done
clear
View Answer play_arrow
question_answer 297) Haemoglobin is having maximum affinity with
A)
\[C{{O}_{2}}\]
done
clear
B)
\[CO\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 298) Veliger larva occurs in phylum
A)
Mollusca
done
clear
B)
Echinodermata
done
clear
C)
Arthropoda
done
clear
D)
Cnidaria
done
clear
View Answer play_arrow
question_answer 299) The most recent and direct prehistoric ancestor of present man is
A)
Cro-magnon
done
clear
B)
Pre-Neanderthal
done
clear
C)
Neanderthal
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 300) Darwins finches refers to
A)
fossils of birds collected by Darwin at Galapagos islands
done
clear
B)
a type of birds present of Galapagos islands
done
clear
C)
migratory birds collected by Darwin at Galapagos islands
done
clear
D)
fossils of reptiles collected by Darwin at Galapagos islands
done
clear
View Answer play_arrow
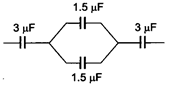
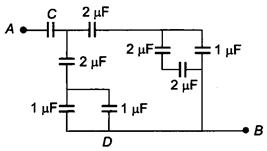
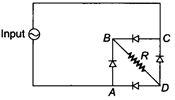
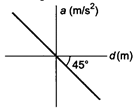
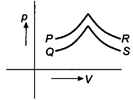
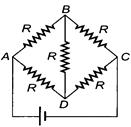
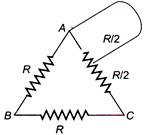
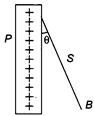
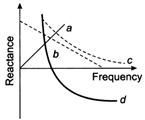
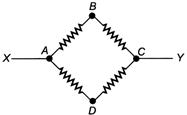
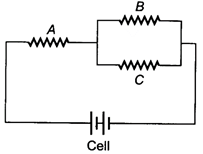
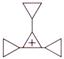 (II)
(II)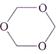 , it is prepared from
, it is prepared from
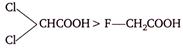 \[>-Cl-C{{H}_{2}}COOH>HCOOH>C{{H}_{3}}COOH\]
\[>-Cl-C{{H}_{2}}COOH>HCOOH>C{{H}_{3}}COOH\]