A) \[{{C}_{2}}{{H}_{2}}\]
B) \[{{C}_{2}}{{H}_{4}}\]
C) \[{{C}_{2}}{{H}_{4}}B{{r}_{2}}\]
D) \[{{C}_{6}}{{H}_{6}}\]
Correct Answer: C
Solution :
As the\[s-\]character or number of TC-electrons increases, bond length decreases. \[H-\underset{sp}{\mathop{C}}\,\overset{2\pi }{\mathop{\equiv }}\,\underset{sp}{\mathop{C}}\,H;\]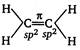
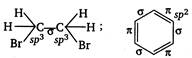 Therefore,\[{{C}_{2}}{{H}_{4}}B{{r}_{2}}\]due to the absence of \[\pi -\]electrons or due to the presence of lesser\[s-\]character (25%), has the longest\[C-H\]bond distance.
Therefore,\[{{C}_{2}}{{H}_{4}}B{{r}_{2}}\]due to the absence of \[\pi -\]electrons or due to the presence of lesser\[s-\]character (25%), has the longest\[C-H\]bond distance.
You need to login to perform this action.
You will be redirected in
3 sec
