A)
![]()
B)
![]()
C)
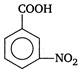
D)
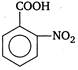
Correct Answer: A
Solution :
Presence of electron withdrawing group increases the acidic strength. It withdraws electrons from the carbon to which it is attached and this effect is transmitted throughout the chain. As a result the electrons are withdrawn more strongly from oxygen of\[O-H\]bond and promotes the release of proton. The o-nitrobenzoic acid is most acidic due to ortho effect while among the p and m-isomers, p-is more acidic because as the distance between\[-N{{O}_{2}}\]group and the\[-COOH\]group increases, the electron withdrawing influence decreases. Therefore, the order of acidic strength is as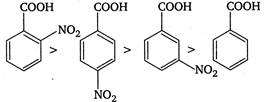 Hence,
Hence,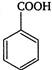 is the weakest acid.
is the weakest acid.
You need to login to perform this action.
You will be redirected in
3 sec
