| \[I.\]\[A\xrightarrow[{}]{Mg/ether}\xrightarrow[(ii){{H}_{3}}{{O}^{+}}]{(i)C{{O}_{2}}}B\] |
| \[II.\]\[A\xrightarrow[{}]{KCN}\xrightarrow[{}]{{{H}_{3}}{{O}^{+}}}B\] |
| \[III.\]\[A\xrightarrow[{}]{Aq.\,KOH}\xrightarrow[{}]{KMn{{O}_{4}}/{{H}^{+}}}B\] |
A) \[I,\text{ }II,\text{ }III\]
B) \[I,\text{ }II\]
C) \[II,\text{ }III\]
D) \[II\]
Correct Answer: B
Solution :
I. \[BrC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\] [A] \[\xrightarrow[{}]{Mg/ether}Br\overset{s+}{\mathop{Mg}}\,\overset{s-}{\mathop{C{{H}_{2}}}}\,C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\]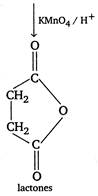
You need to login to perform this action.
You will be redirected in
3 sec
