question_answer 1) A large number of bullets are fired in all directions with same speed v. What is the maximum area on the ground on which these bullets will spread
A)
\[\pi \frac{{{V}^{2}}}{g}\]
done
clear
B)
\[\pi \frac{{{V}^{2}}}{{{g}^{2}}}\]
done
clear
C)
\[{{\pi }^{2}}\frac{{{V}^{4}}}{{{g}^{2}}}\]
done
clear
D)
\[{{\pi }^{2}}\frac{{{V}^{2}}}{{{g}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 2) A force vector applied on a body is given by \[\overset{\text{ }\!\!\hat{\ }\!\!\text{ }}{\mathop{\text{F}}}\,\text{=6}\overset{\text{ }\!\!\hat{\ }\!\!\text{ }}{\mathop{\text{i}}}\,\text{-8}\overset{\text{ }\!\!\hat{\ }\!\!\text{ }}{\mathop{\text{j}}}\,\text{+10}\overset{\text{ }\!\!\hat{\ }\!\!\text{ }}{\mathop{\text{k}}}\,\] and acquires an acceleration of 1 m/s2. Then the mass of the body is
A)
10\[\sqrt{2}\]kg
done
clear
B)
\[2\sqrt{10}\] kg
done
clear
C)
10kg
done
clear
D)
20kg
done
clear
View Answer play_arrow
question_answer 3) A 2 kg block is dropped from a height of 0.4 m on a spring of force constant k = 1960 N/m. The maximum compression of the spring is
A)
0.1 m
done
clear
B)
0.2 m
done
clear
C)
0.3m
done
clear
D)
0.4m
done
clear
View Answer play_arrow
question_answer 4) A solid sphere of mass 2 kg rolls up a \[30{}^\circ \] incline with an initial speed 10 m/s. The maximum height reached by the sphere is\[(g=10m/{{s}^{2}})\]
A)
3.5m
done
clear
B)
7.0m
done
clear
C)
10.Sm
done
clear
D)
14.0m
done
clear
View Answer play_arrow
question_answer 5) The ratio of the adiabatic bulk modulus to the isothermal bulk modulus of a perfect gas is equal to (symbols have their usual meanings)
A)
\[{{C}_{p}}-{{C}_{v}}\]
done
clear
B)
\[\frac{{{C}_{p}}}{{{C}_{v}}}\]
done
clear
C)
\[\frac{{{C}_{v}}}{{{C}_{p}}}\]
done
clear
D)
\[\sqrt{\frac{{{C}_{p}}}{{{C}_{v}}}}\]
done
clear
View Answer play_arrow
question_answer 6) A simple pendulum has time period T. The pendulum is completely immersed in a non-viscous liquid whose density is one-tenth, of that of the material of the bob. The time period of the pendulum immersed in liquid is
A)
\[T\]
done
clear
B)
\[\sqrt{\frac{9}{10}}T\]
done
clear
C)
\[\sqrt{\frac{10}{9}T}\]
done
clear
D)
\[\frac{T}{10}\]
done
clear
View Answer play_arrow
question_answer 7) How many times more intense is a 90 dB sound than a 40 dB sound?
A)
2.5
done
clear
B)
5
done
clear
C)
50
done
clear
D)
10s
done
clear
View Answer play_arrow
question_answer 8) If\[x=\frac{{{\varepsilon }_{0}}lv}{t}\] where \[{{\varepsilon }_{0}}\] is the permittivity of free space, v is length, v is potential difference and t is time. The dimensions of X are the same as that of
A)
charge
done
clear
B)
resistance
done
clear
C)
voltage
done
clear
D)
current
done
clear
View Answer play_arrow
question_answer 9) An electric dipole placed in a uniform electric field will have minimum potential energy when the dipole moment is inclined to the field at an angle
A)
\[\pi \]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
zero
done
clear
D)
\[\frac{3\pi }{2}\]
done
clear
View Answer play_arrow
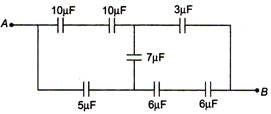
question_answer 10)
In the figure, the equivalent capacitance between A and B is
A)
3.75 \[\pi F\]
done
clear
B)
5.25 \[\pi F\]
done
clear
C)
6.5 \[\pi F\]
done
clear
D)
10.5 \[\pi F\]
done
clear
View Answer play_arrow
question_answer 11) If we add impurity to a metal those atoms also deflect electrons. Therefore,
A)
the electrical and thermal conductivities both increase
done
clear
B)
the electrical and thermal conductivities both decrease
done
clear
C)
the electrical conductivity increases but thermal conductivity decreases
done
clear
D)
the electrical conductivity decreases but thermal conductivity increases
done
clear
View Answer play_arrow
question_answer 12) A toroidal solenoid with an air core has an average radius of 15 cm, area of cross-section 12 cm2 and 1200 turns. Ignoring the field variation across the cross-section of the toroid, the self-inductance of the toroid is
A)
4.6 mH
done
clear
B)
6.9 mH
done
clear
C)
2.3 mH
done
clear
D)
9.2 mH
done
clear
View Answer play_arrow
question_answer 13) Two plane mirrors are placed perpendicular to each other. A ray strikes one mirror and after reflection from the second mirror will be
A)
perpendicular to the original ray
done
clear
B)
parallel to the original ray
done
clear
C)
at\[45{}^\circ \]to the original ray
done
clear
D)
can be at any angle to the original ray
done
clear
View Answer play_arrow
question_answer 14) An electron moving with velocity\[2\times {{10}^{7}}m/s\] describes a circle in a magnetic field of strength\[2\times {{10}^{-2}}T\]. If \[\left( \frac{e}{m} \right)\] of electron is\[1.76\times {{10}^{11}}C/kg\]then the diameter of the circle is nearly
A)
1.1 cm
done
clear
B)
1.1 mm
done
clear
C)
1.1m
done
clear
D)
11cm
done
clear
View Answer play_arrow
question_answer 15) An electron makes transition inside a hydrogen atom. The orbital angular momentum of the electron may change by
A)
\[h\]
done
clear
B)
\[\frac{h}{3\pi }\]
done
clear
C)
\[\frac{h}{2\pi }\]
done
clear
D)
\[\frac{h}{4\pi }\]
done
clear
View Answer play_arrow
question_answer 16) The transfer ratio \[\beta \] of a transistor is 50. The input resistance of the transistor when used in the common emitter mode is 1 \[K\Omega \] The peak value of the collector alternating current for an input peak voltage of 0.01V is
A)
100 \[\mu \]A
done
clear
B)
500 \[\mu \]A
done
clear
C)
0.01 \[\mu \]A
done
clear
D)
0.25 \[\mu \]A
done
clear
View Answer play_arrow
question_answer 17) A combination of convex and concave lenses has power 4 D. If the convex lens has power 5 D, the focal length of the concave lens will be
A)
100cm
done
clear
B)
100 cm
done
clear
C)
\[-1\text{ }cm\]
done
clear
D)
\[\frac{100}{-9}\]cm
done
clear
View Answer play_arrow
question_answer 18) A scooter of mass 120 kg is moving with a uniform velocity of 108 km/h. The force required to stop the vehicle in 10 s is
A)
360 N
done
clear
B)
720 N
done
clear
C)
180 N
done
clear
D)
\[120\times 10.8\text{ }N\]
done
clear
View Answer play_arrow
question_answer 19) A heat engine absorbs heat at\[327{}^\circ C\]and exhausts heat at\[127{}^\circ C\]. The efficiency of engine is \[\eta \] and the maximum amount of work performed by the engine per kilocalorie of heat input is W. Then,
A)
\[\eta \]=0.38
done
clear
B)
\[\eta \]=0.88
done
clear
C)
W=1596J
done
clear
D)
W=1400J
done
clear
View Answer play_arrow
question_answer 20) A simple pendulum with length L and mass of the bob is vibrating with an amplitude a. Then the maximum tension in the string is
A)
mg
done
clear
B)
\[mg\left[ 1+{{\left( \frac{a}{L} \right)}^{2}} \right]\]
done
clear
C)
\[mg{{\left[ 1+\frac{a}{2L} \right]}^{2}}\]
done
clear
D)
mg\[{{\left[ 1+\left( \frac{a}{L} \right) \right]}^{2}}\]
done
clear
View Answer play_arrow
question_answer 21) Two charged conducting spheres of radii \[{{R}_{1}}\] and\[{{R}_{2}}\] separated by a large distance are connected by a long wire. The ratio of the charges on them is
A)
\[\frac{{{R}_{1}}}{{{R}_{2}}}\]
done
clear
B)
\[\frac{{{R}_{2}}}{{{R}_{1}}}\]
done
clear
C)
\[\frac{{{R}_{{{1}^{2}}}}}{{{R}_{{{2}^{2}}}}}\]
done
clear
D)
\[\frac{{{R}_{{{2}^{2}}}}}{{{R}_{{{1}^{2}}}}}\]
done
clear
View Answer play_arrow
question_answer 22) A proton and an a-particle, accelerated through the same potential difference, enter a region of uniform magnetic field normally. If the radius of the proton orbit is 10 cm, then radius of a-orbit is
A)
10cm
done
clear
B)
\[10\sqrt{2}\]cm
done
clear
C)
20cm
done
clear
D)
\[5\sqrt{2}\] cm
done
clear
View Answer play_arrow
question_answer 23) In a noiseless transformer, an alternating current of 2 A is flowing in the primary coil. The number of turns on the primary and secondary coils are 100 and 20 respectively. The value of the current in the secondary coil is
A)
0.08 A
done
clear
B)
0.4 A
done
clear
C)
5 A
done
clear
D)
10 A
done
clear
View Answer play_arrow
question_answer 24) A solenoid 30 cm long is made by winding 2000 loops of wire on an iron rod whose cross-section is 1.5 cm2. If the relative permeability of the iron is 6000, what is the self-inductance of the solenoid?
A)
1.5 H
done
clear
B)
2.5 H
done
clear
C)
3.5 H
done
clear
D)
0.5 H
done
clear
View Answer play_arrow
question_answer 25) A luminous object is placed 20 cm from surface of a convex mirror and plane mirror is set, so that virtual images formed in two mirrors coincide. If plane mirror is at a distance of 12 cm from object, then focal length of convex mirror is
A)
5cm
done
clear
B)
10cm
done
clear
C)
20 cm
done
clear
D)
40 cm
done
clear
View Answer play_arrow
question_answer 26) In a shunted ammeter, only 10% of current passes through the galvanometer of resistance G. The resistance of the shunt is
A)
\[9G\]
done
clear
B)
\[10G\]
done
clear
C)
\[\frac{G}{9}\]
done
clear
D)
\[\frac{G}{10}\]
done
clear
View Answer play_arrow
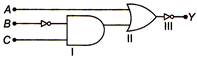
question_answer 27)
The output Y of the logic circuit shown in figure is best represented as
A)
\[\overline{A}+\overline{B.C}\]
done
clear
B)
\[A+\overline{B.}\]
done
clear
C)
\[\overline{A+B.C}\]
done
clear
D)
\[A+\overline{B.}\,C\]
done
clear
View Answer play_arrow
question_answer 28) A resistor of 6 k\[\Omega \] with tolerance 10% and another of 4 k\[\Omega \] with tolerance 10% are connected in series. The tolerance of combination is about
A)
5%
done
clear
B)
10%
done
clear
C)
12%
done
clear
D)
15%
done
clear
View Answer play_arrow
question_answer 29) A stone attached to a rope of length 80 cm is rotated in vertical plane with a speed of 240 rpm. At the moment when the velocity of the stone is directed vertically upwards, the rope ruptures. To what further height does the stone rise? (The air resistance should be neglected)
A)
10.3m
done
clear
B)
41.2m
done
clear
C)
20.4m
done
clear
D)
24.9m
done
clear
View Answer play_arrow
question_answer 30) Two bodies of masses 10 kg and 100 kg are separated by a distance of 2m. The gravitational potential at the mid-point on the line joining the two is
A)
\[7.3\times {{10}^{-7}}\text{J/kg}\]
done
clear
B)
\[7.3\times {{10}^{-8}}\text{J/kg}\]
done
clear
C)
\[7.3\times {{10}^{-9}}\text{J/kg}\]
done
clear
D)
\[7.3\times {{10}^{-6}}\text{J/kg}\]
done
clear
View Answer play_arrow
question_answer 31) A vessel has 6 g of hydrogen at pressure p and temperature 500 K. A small hole is made in it so, That hydrogen leaks out. How much hydrogen leaks out if the final pressure is \[\frac{P}{2}\] and temperature falls to 300 K?
A)
2 g
done
clear
B)
3 g
done
clear
C)
4 g
done
clear
D)
1 g
done
clear
View Answer play_arrow
question_answer 32) For an enclosure maintained at 1000 K, the maximum radiation occurs at wavelength \[{{\lambda }_{m.}}\] If the temperature is raised to 2000 K, the peak will shift to
A)
\[{{\lambda }_{m.}}/2\]
done
clear
B)
\[2{{\lambda }_{m.}}\]
done
clear
C)
\[{{2}^{4}}{{\lambda }_{m.}}\]
done
clear
D)
\[{{2}^{-4}}{{\lambda }_{m.}}\]
done
clear
View Answer play_arrow
question_answer 33) An electron moving in a circular orbit of radius R, with a period T is equivalent to a magnetic dipole of dipole moment
A)
\[\frac{2\pi \operatorname{Re}}{T}\]
done
clear
B)
\[\frac{\pi eR}{T}\]
done
clear
C)
\[\frac{\pi e{{R}^{2}}}{T}\]
done
clear
D)
\[\pi {{R}^{2}}_{e}T\]
done
clear
View Answer play_arrow
question_answer 34) A coil of area\[5c{{m}^{2}}\]and of 20 turns is placed in uniform magnetic field of\[{{10}^{3}}T\]The normal to the plane of the coil makes an angle of\[60{}^\circ \]with the magnetic field. The flux in max well through the coil, is
A)
\[{{10}^{5}}\]
done
clear
B)
\[5\times {{10}^{4}}\]
done
clear
C)
\[2\times {{10}^{4}}\]
done
clear
D)
\[5\times {{10}^{3}}\]
done
clear
View Answer play_arrow
question_answer 35) A coil has an inductance of 0.7 H and is joined in series with a resistance of 220 \[\Omega \] When an alternating emf of 220 Vat 50 cps is applied to it, then the wattless component of the current in the circuit is
A)
5 A
done
clear
B)
0.5 A
done
clear
C)
0.7 A
done
clear
D)
7 A
done
clear
View Answer play_arrow
question_answer 36) An electron jumps from the 4th orbit to the 2nd orbit of hydrogen atom. Given the Rydbergs constant\[R={{10}^{5}}c{{m}^{-1}},\]the frequency in Hz of the emitted radiation will be
A)
\[\frac{3}{10}\times {{10}^{5}}\]
done
clear
B)
\[\frac{16}{3}\times {{10}^{15}}\]
done
clear
C)
\[\frac{9}{16}\times {{10}^{15}}\]
done
clear
D)
\[\frac{3}{4}\times {{10}^{15}}\]
done
clear
View Answer play_arrow
question_answer 37) The decay constant K of a radioactive sample is the probability of decay of an atom in unit time. Then,
A)
\[\lambda \]decreases as the atom becomes older
done
clear
B)
\[\lambda \]increases as the age of atoms increases
done
clear
C)
\[\lambda \]independent of the age of atoms
done
clear
D)
\[\lambda \]behaviour of \[\lambda \]A with time depends on the nature of the activity
done
clear
View Answer play_arrow
question_answer 38) 38. If a surface has work function 4.0 eV, what is the maximum velocity of electrons liberated from the surface when it is irradiated with ultraviolet radiation of wavelength 0.2 \[\mu m\]?
A)
\[4.4\times {{10}^{5}}m/s\]
done
clear
B)
\[8.8\times {{10}^{7}}m/s\]
done
clear
C)
\[8.8\times {{10}^{5}}m/s\]
done
clear
D)
\[4.4\times {{10}^{7}}m/s\]
done
clear
View Answer play_arrow
question_answer 39) An installation consisting of an electric motor driving a water pump lifts 75 L of water per second to a height of 4.7 m. If the motor consumes a power of 5 kW, then the efficiency of the installation is
A)
39%
done
clear
B)
69%
done
clear
C)
93%
done
clear
D)
96%
done
clear
View Answer play_arrow
question_answer 40) The Keplers second law states that the straight line joining the planet to the sun sweeps out equal areas in equal times. The statement is equivalent to saying that
A)
total acceleration is zero
done
clear
B)
transverse acceleration is zero
done
clear
C)
longitudinal acceleration is zero
done
clear
D)
radial acceleration is zero
done
clear
View Answer play_arrow
question_answer 41) The number of possible structures of amines\[({{C}_{7}}{{H}_{9}}N)\]having one benzene ring is
A)
5
done
clear
B)
3
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 42) Vitamin\[{{B}_{1}}\]is
A)
riboflavin
done
clear
B)
cobalamin
done
clear
C)
thiamine
done
clear
D)
pyridoxine
done
clear
View Answer play_arrow
question_answer 43) During roasting of zinc blende, it converts to
A)
\[ZnO\]
done
clear
B)
\[ZnS{{O}_{4}}\]
done
clear
C)
\[ZnC{{O}_{3}}\]
done
clear
D)
\[Zn\]
done
clear
View Answer play_arrow
question_answer 44) \[Xe{{F}_{4}}\]reacts with water at\[-80{}^\circ C\]to give
A)
\[XeO{{F}_{2}}\]
done
clear
B)
\[XeO{{F}_{4}}\]
done
clear
C)
\[Xe{{O}_{3}}\]
done
clear
D)
\[Xe{{O}_{2}}{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 45) The number of\[POP\]bonds in the structure of phosphorus pentaoxide and phosphorus trioxide are respectively
A)
6,6
done
clear
B)
5,5
done
clear
C)
5, 6
done
clear
D)
6, 5
done
clear
View Answer play_arrow
question_answer 46) The most unsymmetrical and symmetrical systems are respectively
A)
tetragonal, cubic
done
clear
B)
triclinic, cubic
done
clear
C)
rhombohedral, hexagonal
done
clear
D)
orthorhombic, cubic
done
clear
View Answer play_arrow
question_answer 47) In the reaction,\[HN{{O}_{3}}\]+Rpio\[\xrightarrow[{}]{{}}\]\[4HP{{O}_{3}}+x,\]the product X is
A)
\[N{{O}_{2}}\]
done
clear
B)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 48) The basicity of orthophosphoric acid is
A)
2
done
clear
B)
4
done
clear
C)
3
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 49) In the radioactive change, \[_{z}{{p}^{A}}{{\xrightarrow[{}]{{}}}_{z+1}}{{Q}^{A}}{{\xrightarrow[{}]{{}}}_{z-1}}{{R}^{A-4}}{{\xrightarrow[{}]{{}}}_{z-1}}{{S}^{A-4}},\] the radiations emitted in sequence are
A)
\[\alpha ,\beta ,\gamma \]
done
clear
B)
\[\beta ,\alpha ,\gamma \]
done
clear
C)
\[\gamma ,\alpha ,\beta \]
done
clear
D)
\[\beta ,\gamma ,\alpha \]
done
clear
View Answer play_arrow
question_answer 50) In contact process of manufacture\[{{H}_{2}}S{{O}_{4}},\]the catalyst used is
A)
iron
done
clear
B)
\[{{V}_{2}}{{O}_{5}}\]
done
clear
C)
chromium
done
clear
D)
oxides of nitrogen
done
clear
View Answer play_arrow
question_answer 51) Which of the following is pseudohalogen?
A)
\[I{{F}_{7}}\]
done
clear
B)
\[{{(CN)}_{2}}\]
done
clear
C)
\[IC{{l}_{2}}\]
done
clear
D)
\[I_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 52) Correct formula of calomel is
A)
\[HgC{{l}_{2}}\]
done
clear
B)
\[HgC{{l}_{2}}.{{H}_{2}}O\]
done
clear
C)
\[H{{g}_{2}}C{{l}_{2}}\]
done
clear
D)
\[HgS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 53) The angle in case of a water molecule is approximately
A)
\[90{}^\circ \]
done
clear
B)
\[180{}^\circ \]
done
clear
C)
\[105{}^\circ \]
done
clear
D)
\[75{}^\circ \]
done
clear
View Answer play_arrow
question_answer 54) Which one is an example of globular protein?
A)
Keratin
done
clear
B)
Collagen
done
clear
C)
Insulin
done
clear
D)
Myosin
done
clear
View Answer play_arrow
question_answer 55) Which of the following reaction takes place when a mixture of concentrated\[HN{{O}_{3}}\]and \[{{H}_{2}}S{{O}_{4}}\]reacts with benzene at 350K?
A)
Sulphonation
done
clear
B)
Nitration
done
clear
C)
Hydrogenation
done
clear
D)
Dehydration
done
clear
View Answer play_arrow
question_answer 56) How many chiral carbon atoms are present in 2, 3, 4 - Trichloropentane?
A)
Three
done
clear
B)
Two
done
clear
C)
One
done
clear
D)
Four
done
clear
View Answer play_arrow
question_answer 57) Geometry of reaction intermediate in\[{{S}_{N}}1\]reaction is
A)
tetrahedral
done
clear
B)
planar
done
clear
C)
triangular bipyramidal
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 58) In acetylene molecule, the two carbon atoms are linked by
A)
One-o-bond and two Ti-bonds
done
clear
B)
two o-bonds and one TC-bond
done
clear
C)
three o-bonds
done
clear
D)
three TC-bonds
done
clear
View Answer play_arrow
question_answer 59) In a galvanic cell, which one of the following statements is not correct?
A)
Anode is negatively charged
done
clear
B)
Cathode is positively charged
done
clear
C)
Reduction takes place at the anode
done
clear
D)
Reduction takes place at the cathode
done
clear
View Answer play_arrow
question_answer 60) The zig-zag motion of colloidal particles was first observed by
A)
John Tyndall
done
clear
B)
Robert Brown
done
clear
C)
Zsigmondy
done
clear
D)
Ostwald
done
clear
View Answer play_arrow
question_answer 61) Which of the following is not present in DNA?
A)
Adenine
done
clear
B)
Guanine
done
clear
C)
Uracil
done
clear
D)
Thymine
done
clear
View Answer play_arrow
question_answer 62) Oxygen and ozone are
A)
isotopes
done
clear
B)
isomers
done
clear
C)
isobars
done
clear
D)
allotropes
done
clear
View Answer play_arrow
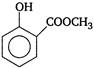
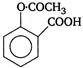
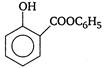
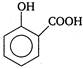
question_answer 63) Which of the following is weakest acid?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 64) The reaction between an alkyl halide and an alkoxide ion to give an ether is known as
A)
Williamsons synthesis
done
clear
B)
Clemmensens reduction
done
clear
C)
Fittig reaction
done
clear
D)
Wurtz reaction
done
clear
View Answer play_arrow
question_answer 65)
A)
\[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,OC{{H}_{2}}C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}O\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,C{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 66) \[[CoBr{{(N{{H}_{3}})}_{5}}]S{{O}_{4}}\]and\[[CoS{{O}_{4}}{{(N{{H}_{3}})}_{5}}]Br\]are related to each other as
A)
ionisation isomers
done
clear
B)
linkage isomers
done
clear
C)
coordinatiom isomers
done
clear
D)
optical isomiers
done
clear
View Answer play_arrow
question_answer 67) Which of the following atoms has no neutron in its nucleus?
A)
Helium
done
clear
B)
Lithium
done
clear
C)
Protium
done
clear
D)
Tritium
done
clear
View Answer play_arrow
question_answer 68) With increasing bond order, stability of a bond
A)
increases
done
clear
B)
decreases
done
clear
C)
remains unaltered
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 69) Urotropine is obtained by the reaction of\[N{{H}_{3}}\]with
A)
\[C{{H}_{3}}OH\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 70) Wolff-Kishner reaction is used for the reduction of
A)
carboxylic acids
done
clear
B)
carbonyl compounds
done
clear
C)
olefins
done
clear
D)
nitro compounds
done
clear
View Answer play_arrow
question_answer 71)
4-bromo-l-butanol [A] can be converted into 5-hydroxy pentanoic acid [B] by following method [s] \[I.\]\[A\xrightarrow[{}]{Mg/ether}\xrightarrow[(ii){{H}_{3}}{{O}^{+}}]{(i)C{{O}_{2}}}B\] \[II.\]\[A\xrightarrow[{}]{KCN}\xrightarrow[{}]{{{H}_{3}}{{O}^{+}}}B\] \[III.\]\[A\xrightarrow[{}]{Aq.\,KOH}\xrightarrow[{}]{KMn{{O}_{4}}/{{H}^{+}}}B\]
Select the correct alternate
A)
\[I,\text{ }II,\text{ }III\]
done
clear
B)
\[I,\text{ }II\]
done
clear
C)
\[II,\text{ }III\]
done
clear
D)
\[II\]
done
clear
View Answer play_arrow
question_answer 72) Oil of winter green is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 73) \[\alpha -D-\]glucose and\[\beta -D-\]glucose have a specific rotation of\[+112{}^\circ \]and\[+19.2{}^\circ \] respectively. In aqueous solution, the rotation becomes\[+52{}^\circ \]. This is known as
A)
racemisation
done
clear
B)
mutarotation
done
clear
C)
inversion
done
clear
D)
enolisation
done
clear
View Answer play_arrow
question_answer 74) Time required to decompose\[S{{O}_{2}}C{{l}_{2}}\]to half of its initial amount is 60 min. If the decomposition is a first order reaction, what is the rate constant of the reaction?
A)
\[1.925\times {{10}^{-4}}s\]
done
clear
B)
\[2.092\times {{10}^{-4}}{{s}^{-1}}\]
done
clear
C)
\[1.925\times {{10}^{-4}}{{s}^{-1}}\]
done
clear
D)
\[2.925\times {{10}^{-4}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following has a bond order of 1.75?
A)
\[ClO_{3}^{-}\]
done
clear
B)
\[ClO_{4}^{-}\]
done
clear
C)
\[NO_{3}^{-}\]
done
clear
D)
\[CO_{3}^{2-}\]
done
clear
View Answer play_arrow
question_answer 76) The ionisation energy of nitrogen is larger than that of oxygen because of
A)
greater attraction of electrons by the nucleus
done
clear
B)
the size of nitrogen atom being smaller
done
clear
C)
the half-filled p-orbitals possess extra stability
done
clear
D)
greater penetration effect
done
clear
View Answer play_arrow
question_answer 77) Which one is the strongest reducing agent?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[As{{H}_{3}}\]
done
clear
C)
\[Sb{{H}_{3}}\]
done
clear
D)
\[P{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 78) Preparation of ethane by electrolysis of aqueous solution of potassium acetate is called
A)
Wurtz reaction
done
clear
B)
Kolbes electrolysis
done
clear
C)
Grignard reaction
done
clear
D)
Sabatier-Sendersens reaction
done
clear
View Answer play_arrow
question_answer 79) What is density of\[S{{O}_{2}}\]gas at\[27{}^\circ C\]and 2 arm pressure? (Atomic weight\[S=32,\text{ O}=16,\] \[R=0.0821\text{ }L\text{ }atm\text{ }{{K}^{-1}}mo{{l}^{-1}}\])
A)
5.0246
done
clear
B)
4.1246
done
clear
C)
3.0196
done
clear
D)
5.1969
done
clear
View Answer play_arrow
question_answer 80) 38.00 mL of moist nitrogen gas were collected at\[27{}^\circ C\]and 746.5 mm pressure. What is the volume of the gas at\[0{}^\circ C\]and 760 mm pressure? (Aq. tension at\[27{}^\circ C\]is 26.5 mm).
A)
32.76
done
clear
B)
23.76
done
clear
C)
12.05
done
clear
D)
11.56
done
clear
View Answer play_arrow
question_answer 81) What is the enthalpy of hydrogenation of ethylene, given that the enthalpy of combustion of ethylene, hydrogen and ethane are\[-1410.0,-286.2\]and\[-1560.6\text{ }kJ\text{ }mo{{l}^{-1}}\]respectively at 298 K?
A)
135.6
done
clear
B)
\[-135.6\]
done
clear
C)
125.6
done
clear
D)
\[-125.6\]
done
clear
View Answer play_arrow
question_answer 82) What is the pH value of\[0.03\text{ }N\text{ }HCl\]solution?
A)
2.5229
done
clear
B)
3.2229
done
clear
C)
1.5229
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 83) The\[{{H}_{2}}{{O}^{+}}\]ion concentration of a solution having pH 6.58 is
A)
\[2.63\times {{10}^{7}}\]
done
clear
B)
\[2.63\times {{10}^{-7}}\]
done
clear
C)
\[36.2\times {{10}^{7}}\]
done
clear
D)
\[36.2\times {{10}^{-7}}\]
done
clear
View Answer play_arrow
question_answer 84) One mole of magnesium nitride on reaction with an excess of water gives
A)
one mole of ammonia
done
clear
B)
one mole of nitric acid
done
clear
C)
two moles of ammonia
done
clear
D)
two moles of nitric acid
done
clear
View Answer play_arrow
question_answer 85) Photoelectric effect is maximum in
A)
Cs
done
clear
B)
Na
done
clear
C)
K
done
clear
D)
\[Li\]
done
clear
View Answer play_arrow
question_answer 86) Fused ionic compounds
A)
are insulators
done
clear
B)
are used as semiconductors
done
clear
C)
conduct electricity
done
clear
D)
do not conduct electricity
done
clear
View Answer play_arrow
question_answer 87) The bonds between P atoms and\[Cl\]atoms in\[PC{{l}_{5}}\]are likely to be
A)
ionic with no covalent character
done
clear
B)
covalent with some ionic character
done
clear
C)
covalent with no ionic character
done
clear
D)
ionic with some metallic character
done
clear
View Answer play_arrow
question_answer 88) Covalent compounds are soluble in
A)
polar solvents
done
clear
B)
non-polar solvents
done
clear
C)
concentrated acids
done
clear
D)
all solvents
done
clear
View Answer play_arrow
question_answer 89) An ingredient of baking powder is
A)
sodium bicarbonate
done
clear
B)
sodium chloride
done
clear
C)
borax
done
clear
D)
sodium carbonate
done
clear
View Answer play_arrow
question_answer 90) The most reactive allotrope of phosphorus is
A)
violet phosphorus
done
clear
B)
white phosphorus
done
clear
C)
red phosphorus
done
clear
D)
scarlet phosphorus
done
clear
View Answer play_arrow
question_answer 91) Correct order representing electro negativities of halogens is
A)
\[F<Cl<Br<I\]
done
clear
B)
\[F>Cl>Br>I\]
done
clear
C)
\[F>Cl<Br<I\]
done
clear
D)
\[F<Cl>Br>I\]
done
clear
View Answer play_arrow
question_answer 92) For a first order reaction,\[A\to \]products, if k is the rate constant then the half-life period of the reaction is
A)
\[\frac{1}{k}\log \frac{[A]}{{{[A]}_{0}}}\]
done
clear
B)
\[\frac{1}{k}\frac{1}{[A]}\]
done
clear
C)
\[\frac{0.693}{k}\]
done
clear
D)
\[\frac{\log 2}{k}\]
done
clear
View Answer play_arrow
question_answer 93) Dead burnt plaster is
A)
\[CaS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
B)
\[MgS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
C)
\[CaS{{O}_{4}}.1/2{{H}_{2}}O\]
done
clear
D)
\[CaS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 94) The active constituent of bleaching powder is
A)
\[Ca{{(OCl)}_{2}}\]
done
clear
B)
\[Ca(OCl)Cl\]
done
clear
C)
\[Ca{{(Cl{{O}_{2}})}_{2}}\]
done
clear
D)
\[Ca(Cl{{O}_{2}})Cl\]
done
clear
View Answer play_arrow
question_answer 95) Which of the following is applied in this reaction? \[C{{H}_{3}}CHBrC{{H}_{2}}C{{H}_{3}}\xrightarrow[{}]{Alc.\,KOH}\] (i) \[C{{H}_{3}}CH=CHC{{H}_{3}}\]major product (ii)\[C{{H}_{2}}=CHC{{H}_{2}}C{{H}_{3}}\]minor product
A)
Hermanns rule
done
clear
B)
Kharasch effect
done
clear
C)
Saytzeffs rule
done
clear
D)
Markownikoffs rule
done
clear
View Answer play_arrow
question_answer 96) Which of the following compound is unknown?
A)
\[NC{{l}_{5}}\]
done
clear
B)
\[N{{l}_{3}}\]
done
clear
C)
\[SbC{{l}_{3}}\]
done
clear
D)
\[NC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 97) Group displacement law was given by
A)
Becquerel
done
clear
B)
Rutherford
done
clear
C)
Soddy and Fajan
done
clear
D)
Madam Curie
done
clear
View Answer play_arrow
question_answer 98) Which of the following represents the Lewis structure of\[{{N}_{2}}\]molecule?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 99) Cellulose is a polymer containing the monomer
A)
ribose
done
clear
B)
glucose
done
clear
C)
fructose
done
clear
D)
starch
done
clear
View Answer play_arrow
question_answer 100) Minamata disease is due to pollution of
A)
organic waste into drinking water
done
clear
B)
oil spill in water
done
clear
C)
industrial waste mercury into fishing water
done
clear
D)
arsenic into the atmosphere
done
clear
View Answer play_arrow
question_answer 101) The viruses with nucleic acid but without protein coat are known as
A)
viroids
done
clear
B)
virions
done
clear
C)
capsomeres
done
clear
D)
prions
done
clear
View Answer play_arrow
question_answer 102) Identify the vector suitable to clone long fragments of DNA.
A)
Phage vectors
done
clear
B)
Bacterial plasmids
done
clear
C)
Yeast plasmids
done
clear
D)
Cosmids
done
clear
View Answer play_arrow
question_answer 103) Genetic recombination by transduction in bacterial was discovered first in
A)
Salmonella typhimurium
done
clear
B)
Escherichia coli
done
clear
C)
Streptococcus pneumoniae
done
clear
D)
Agrobactermm tumefaciens
done
clear
View Answer play_arrow
question_answer 104) The brown hair present at the base of the petiole of Pteris are called
A)
setae
done
clear
B)
ramenta
done
clear
C)
spines
done
clear
D)
stipules
done
clear
View Answer play_arrow
question_answer 105) Number of peristomial teeth in Funaria capsule is
A)
16
done
clear
B)
32
done
clear
C)
64
done
clear
D)
128
done
clear
View Answer play_arrow
question_answer 106) Development of haploid plants from totipotent pollen is called
A)
androgenesis
done
clear
B)
parthenocarpy
done
clear
C)
emasculation
done
clear
D)
somatic hybridization
done
clear
View Answer play_arrow
question_answer 107) Grass stem elongates after initial growth due to
A)
lateral meristem
done
clear
B)
secondary meristem
done
clear
C)
intercalary meristem
done
clear
D)
apical meristem
done
clear
View Answer play_arrow
question_answer 108) Protoplast lacks
A)
cytoplasm
done
clear
B)
nucleus
done
clear
C)
plasma membrane
done
clear
D)
cell wall
done
clear
View Answer play_arrow
question_answer 109) An example of physiological xerophyte is
A)
Salvinia
done
clear
B)
Euphorbia
done
clear
C)
Salicornia
done
clear
D)
Agave
done
clear
View Answer play_arrow
question_answer 110) Blood glands of Pheretima are located in which segments?
A)
1, 2 and 3
done
clear
B)
3, 4 and 5
done
clear
C)
4, 5 and 6
done
clear
D)
10, 11 and 12
done
clear
View Answer play_arrow
question_answer 111) Silicates consumed along with food by Pheretima and excreted by
A)
flame cells
done
clear
B)
chloragogen cells
done
clear
C)
intestinal cells
done
clear
D)
basal cells
done
clear
View Answer play_arrow
question_answer 112) Association between barnacles and Limulus is
A)
symbiosis
done
clear
B)
antagonism
done
clear
C)
ectocommens.alism
done
clear
D)
endocommensalism
done
clear
View Answer play_arrow
question_answer 113) The cells are chlorophyllous, fewer in number, unique in shape and inner walls thick. This decription fits into
A)
subsidiary cells
done
clear
B)
guard cells
done
clear
C)
passage cells
done
clear
D)
bulliform cells
done
clear
View Answer play_arrow
question_answer 114) The scientific name of lung fluke of man is
A)
Hymenolepis nana
done
clear
B)
Schistosomum haematobium
done
clear
C)
Paragonimus westermani
done
clear
D)
Echinococcus granulosus
done
clear
View Answer play_arrow
question_answer 115) Plasmodium falciparum causes which type of malaria?
A)
Quartan
done
clear
B)
Pernicious
done
clear
C)
Tertian
done
clear
D)
Benign tertian
done
clear
View Answer play_arrow
question_answer 116) The structures which join two bones at joints are made up of
A)
elastic fibres
done
clear
B)
collagen fibres
done
clear
C)
fibrocytes
done
clear
D)
chondrocytes
done
clear
View Answer play_arrow
question_answer 117) Development of mesoderm in the form of muscles in body wall, leaving alimentary canal non-muscular is the feature of
A)
acoelomates
done
clear
B)
pseudocoelomates
done
clear
C)
enterocoelomates
done
clear
D)
schizocoelomates
done
clear
View Answer play_arrow
question_answer 118) Dermatobiasis in cattle is caused by
A)
maggots of bot fly
done
clear
B)
wriggler of mosquito
done
clear
C)
nits of head louse
done
clear
D)
drones of honey bee
done
clear
View Answer play_arrow
question_answer 119) The type of vascular bundles in the stem of Dracaena is
A)
radial
done
clear
B)
bicollateral
done
clear
C)
amphicribal
done
clear
D)
amphivasal
done
clear
View Answer play_arrow
question_answer 120) Schuffners dots produced by Plasmodium are
A)
reserve food
done
clear
B)
antibodies
done
clear
C)
hormones
done
clear
D)
antigens
done
clear
View Answer play_arrow
question_answer 121) Which of the following has thorns bearing leaves and flowers on them?
A)
Duranta
done
clear
B)
Artabotrys
done
clear
C)
Carissa
done
clear
D)
Bougainvillea
done
clear
View Answer play_arrow
question_answer 122) When malic acid is the respiratory substrate
A)
the amount of carbon dioxide released is more than the oxygen consumed
done
clear
B)
the amount of carbon dioxide released is less than the oxygen consumed
done
clear
C)
the amount of carbon dioxide released is equal to oxygen consumed
done
clear
D)
carbon dioxide is not released
done
clear
View Answer play_arrow
question_answer 123) The risk of spoilage is less in salted pickles because of
A)
guttation
done
clear
B)
plasmolysis
done
clear
C)
imbibition
done
clear
D)
diffusion
done
clear
View Answer play_arrow
question_answer 124) In photosynthesis electron transport system, manganese ions are associated with
A)
oxygen evolving complex
done
clear
B)
cytochrome \[{{f}_{0}}-{{f}_{1}}\]
done
clear
C)
cytochromehy bg \[{{b}_{6}}-f\]complex
done
clear
D)
plastoquinones
done
clear
View Answer play_arrow
question_answer 125) Which of the following is needed both in photosynthesis and respiration?
A)
Chlorophyll
done
clear
B)
Carbon dioxide
done
clear
C)
Oxygen
done
clear
D)
Cytochrome
done
clear
View Answer play_arrow
question_answer 126) Identify the trisaccharide
A)
Mannose
done
clear
B)
Maltose
done
clear
C)
Raffinose
done
clear
D)
Galactose
done
clear
View Answer play_arrow
question_answer 127) Karyotype means a
A)
single haploid set of chromosomes of an organisms
done
clear
B)
diagrammatic representation of the chromosome
done
clear
C)
genes constituting a single set of chromosomes
done
clear
D)
type of nucleus of an organism
done
clear
View Answer play_arrow
question_answer 128) The underground stem of this genus is a rhizome
A)
Allium
done
clear
B)
Gloriosa
done
clear
C)
Scilla
done
clear
D)
Lilium
done
clear
View Answer play_arrow
question_answer 129) The characteristic fruit type in Asteraceae is
A)
carcerulus
done
clear
B)
capsule
done
clear
C)
cypsela
done
clear
D)
caryopsis
done
clear
View Answer play_arrow
question_answer 130) Mode of feeding in tunicates
A)
acrophagus
done
clear
B)
parasitic
done
clear
C)
ciliary or filter
done
clear
D)
myxotrophic
done
clear
View Answer play_arrow
question_answer 131) Valve of Thebesius is present in the heart of
A)
fish
done
clear
B)
frog
done
clear
C)
Chamaeleon
done
clear
D)
rabbit
done
clear
View Answer play_arrow
question_answer 132) Soluble fibrinogen is converted into insoluble fibrin by the action of
A)
thromboplastin
done
clear
B)
cephalin
done
clear
C)
heparin
done
clear
D)
thrombin
done
clear
View Answer play_arrow
question_answer 133) Silicosis is caused by
A)
acid rain
done
clear
B)
depletion of ozone
done
clear
C)
inhalation of aerosols
done
clear
D)
inhalation of sulphur dioxide
done
clear
View Answer play_arrow
question_answer 134) The plasmid \[_{\text{p}}\text{B}{{\text{R}}_{\text{322}}}\] used in biotechnology is
A)
yeast
done
clear
B)
\[{{\text{M}}^{\text{32}}}\]phage
done
clear
C)
parasite
done
clear
D)
cloning vehicle
done
clear
View Answer play_arrow
question_answer 135) In Cycas, diploxylic condition is found in
A)
stem
done
clear
B)
root
done
clear
C)
coralloid root
done
clear
D)
leaflet
done
clear
View Answer play_arrow
question_answer 136) The cyst wall of Euglena is made up of
A)
carbohydrates
done
clear
B)
lipoproteins
done
clear
C)
lipids
done
clear
D)
histones
done
clear
View Answer play_arrow
question_answer 137) Western blot test is done for the conformation of
A)
malaria
done
clear
B)
filaria
done
clear
C)
anaemia
done
clear
D)
AIDS
done
clear
View Answer play_arrow
question_answer 138) Separation of sick and non-productive birds from healthy and productive birds is known as
A)
deworming
done
clear
B)
culling
done
clear
C)
dubbing
done
clear
D)
cannibalism
done
clear
View Answer play_arrow
question_answer 139) In Spirogyra, a brief period of tetranucleate condition is found in
A)
hold fast
done
clear
B)
gametangium
done
clear
C)
vegetative cell
done
clear
D)
germinating zygote
done
clear
View Answer play_arrow
question_answer 140) An example for holandric inheritance is
A)
epidermolysis
done
clear
B)
Turners syndrome
done
clear
C)
haemophilia
done
clear
D)
webbed toes
done
clear
View Answer play_arrow
question_answer 141) For a gene with two alleles, if the gene frequency of recessive gene is 0.2. What is the genotypic frequency of homozygous dominant?
A)
0.064
done
clear
B)
0.8
done
clear
C)
0.64
done
clear
D)
0.32
done
clear
View Answer play_arrow
question_answer 142) The number of lobes in left lung of rabbit is
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
five
done
clear
View Answer play_arrow
question_answer 143) When one gene influences the expression of another non-allelic gene, it is termed as
A)
epistasis
done
clear
B)
poly multiple allelism
done
clear
C)
segregation
done
clear
D)
pleiotropy
done
clear
View Answer play_arrow
question_answer 144) An example of sex-influenced inheritance is
A)
haemophilia
done
clear
B)
colour blindness
done
clear
C)
baldness
done
clear
D)
Downs syndrome
done
clear
View Answer play_arrow
question_answer 145) Which one of the following is a reference to xenogamy?
A)
Ripening of androecium earlier to gynoecium
done
clear
B)
Pollen grains of one flower reaching the stigma of another flower present on the same plant
done
clear
C)
Pollen grains of one flower, reaching the stigma of another flower present on a different plant of the same species
done
clear
D)
The inability of pollen to germinate on the stigma of the same flower
done
clear
View Answer play_arrow
question_answer 146) Which one of the following is useful in identifying the different strains of causal microbe of an infectious disease?
A)
Colchicine
done
clear
B)
Agrobacterium
done
clear
C)
Complementary DNA
done
clear
D)
Crystal violet
done
clear
View Answer play_arrow
question_answer 147) The photoperiodic cycles of 6 hours of dark period and 18 hours of light period induce flower formation in
A)
Xanthium
done
clear
B)
tobacco
done
clear
C)
soybean
done
clear
D)
Beta vulgaris
done
clear
View Answer play_arrow
question_answer 148) When one molecule of glucose is completely oxidised during aerobic respiration, how many molecules of carbon dioxide are released due to tricarboxylic acid cycle?
A)
One
done
clear
B)
Two
done
clear
C)
Three
done
clear
D)
Four
done
clear
View Answer play_arrow
question_answer 149) Which of the following reaction does not take place in the cell organelle, that is referred to as power house of the cell?
A)
Glycine decarboxylation
done
clear
B)
Glyceraldehyde 3-phosphate drogenation
done
clear
C)
Fumaric acid hydration
done
clear
D)
Cytochrome oxidation
done
clear
View Answer play_arrow
question_answer 150) Swollen and spongy petioles are characteristic of
A)
Trapa
done
clear
B)
Wolffia
done
clear
C)
Ceratophyllum
done
clear
D)
Limnophila
done
clear
View Answer play_arrow
question_answer 151) A plant with low \[C{{O}_{2}}\] compensation point is
A)
Atriplex patula
done
clear
B)
Leucopoa kingu
done
clear
C)
Gossypium hirsutum
done
clear
D)
Tidestromia oblongifolia
done
clear
View Answer play_arrow
question_answer 152) During which phase of their replication, the bacteriphages release lysozyme?
A)
Adsorption
done
clear
B)
Maturation
done
clear
C)
Eclipse
done
clear
D)
Penetration
done
clear
View Answer play_arrow
question_answer 153) In Pheretima, the number of ring vessels per segment in 12th and 13th segment, is
A)
10 pairs
done
clear
B)
10 pairs
done
clear
C)
12 pairs
done
clear
D)
24 pairs
done
clear
View Answer play_arrow
question_answer 154) A couple whose sons are colour blind with AB blood group, identify the parent from the following.
A)
Mother colourblind with blood group-A and father normal with blood group-B
done
clear
B)
Mother normal with blood group-A and father colourblind with blood group-B
done
clear
C)
Mother colourblind with blood group-B and father normal with blood group-B
done
clear
D)
Mother normal with blood group-A and father colourblind with blood group-B
done
clear
View Answer play_arrow
question_answer 155) An example of vestigial organ is
A)
wing of ApteryJc
done
clear
B)
tail of Macropus
done
clear
C)
eyelid of man
done
clear
D)
flipper of whale
done
clear
View Answer play_arrow
question_answer 156) In a cross between individuals with genotypes TrRr, if the resulting number of off springs is 16, identify the number of genotypes with TtRr and TtRR amongst them.
A)
1 and 2
done
clear
B)
2 and 3
done
clear
C)
3 and 1
done
clear
D)
4 and 2
done
clear
View Answer play_arrow
question_answer 157) During oxygen transport, the oxyhaemoglobin at the tissue level liberates oxygen to the cells due to
A)
\[{{O}_{2}}\] concentration is high and \[C{{O}_{2}}\] is low
done
clear
B)
\[{{O}_{2}}\] concentration is low and \[C{{O}_{2}}\] is high
done
clear
C)
\[{{O}_{2}}\] tension is low and \[C{{O}_{2}}\] tension is high
done
clear
D)
\[{{O}_{2}}\] tension is high and \[C{{O}_{2}}\] tension is low
done
clear
View Answer play_arrow
question_answer 158) The gestation period of cow is
A)
30 days
done
clear
B)
270 days
done
clear
C)
280 days
done
clear
D)
300 days
done
clear
View Answer play_arrow
question_answer 159) In protein synthesis, the initiator codon is
A)
AAA
done
clear
B)
AGC
done
clear
C)
AAT
done
clear
D)
AUG
done
clear
View Answer play_arrow
question_answer 160) Adaptive radiation is an example of
A)
directional selection
done
clear
B)
diversifying selection
done
clear
C)
stabilising selection
done
clear
D)
sympatric speciation
done
clear
View Answer play_arrow
question_answer 161) What percentage of homozygous \[R{{h}^{-}}\] will be born amongst 4 children couple where the husband is heterozygous for \[R{{h}^{+}}\]and wife is homozygous for \[R{{h}^{-}}\] gene?
A)
25%
done
clear
B)
50%
done
clear
C)
75%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 162) One of the human diseases due to magnification of heavy metal is
A)
Minimata
done
clear
B)
asthma
done
clear
C)
tuberculosis
done
clear
D)
elephantiasis
done
clear
View Answer play_arrow
question_answer 163) The ears of a mammal living in a cold area were smaller than the one living in a warm area. This is an example of
A)
Bergmans rule
done
clear
B)
Jordans rule
done
clear
C)
Aliens rule
done
clear
D)
Glogers rule
done
clear
View Answer play_arrow
question_answer 164) The growth rate of population stabilises after
A)
logarithmic phase
done
clear
B)
stationary phase
done
clear
C)
carrying capacity
done
clear
D)
negative acceleration phase
done
clear
View Answer play_arrow
question_answer 165) Identify from the following which can give a complementary and palindromic sequence.
A)
5ATATCC3
done
clear
B)
5 CCGAAT 3
done
clear
C)
5GAATTCS
done
clear
D)
3AGGTTCS
done
clear
View Answer play_arrow
question_answer 166) According to IUGN red list, what is the status of red panda (Athurus fulgens)?
A)
Vulnerable species
done
clear
B)
Critically endangered species
done
clear
C)
Extinct species
done
clear
D)
Endangered species
done
clear
View Answer play_arrow
question_answer 167) In which one pair, both the plants can be vegetatively propagated by leaf pieces?
A)
Bryophyllum and Kalanchoe
done
clear
B)
Chrysanthemum and Agave
done
clear
C)
Agave and Kalanchoe
done
clear
D)
Asparagus and Bryophyllum
done
clear
View Answer play_arrow
question_answer 168) G-6-P dehydrogenase deficiency is associated with haemolysis of
A)
lymphocytes
done
clear
B)
RBCs
done
clear
C)
platelets
done
clear
D)
leucocytes
done
clear
View Answer play_arrow
question_answer 169) The name of Norman Borlaug is associated with
A)
green revolution
done
clear
B)
yellow revolution
done
clear
C)
white revolution
done
clear
D)
blue revolution
done
clear
View Answer play_arrow
question_answer 170) Which one of the following makes use of RNA as a template to synthesise DNA?
A)
Reverse transcriptase
done
clear
B)
DNA dependent RNA polymerase
done
clear
C)
DNA polymerase
done
clear
D)
RNA polymerase
done
clear
View Answer play_arrow
question_answer 171) One of the most important function of botanical gardens is that
A)
one can observe tropical plant there
done
clear
B)
they allow ex situ conservation of germplasm
done
clear
C)
they provide the natural habitat for wildlife
done
clear
D)
they provide a beautiful area for recreation
done
clear
View Answer play_arrow
question_answer 172) Protein synthesis in an animal cell occurs
A)
only on the ribosomes present in cytosol
done
clear
B)
on ribosomes present in cytoplasm as well as in mitochondria
done
clear
C)
only on ribosomes attached to the nuclear envelope and endoplasmic reticulum
done
clear
D)
on ribosomes present in the nucleolus as well as in cytoplasm
done
clear
View Answer play_arrow
question_answer 173) Which of the following is not true for a species?
A)
Members of a species can interbreed
done
clear
B)
Variations occurs among members of a species
done
clear
C)
Each species is reproductively isolated from every other species
done
clear
D)
Gene flow does not occur between the populations of a species
done
clear
View Answer play_arrow
question_answer 174) Potometer works on the principle of
A)
amount of water absorbed equals the amount transpired
done
clear
B)
osmotic pressure
done
clear
C)
root pressure
done
clear
D)
potential difference between the tip of the tube and that of the plant
done
clear
View Answer play_arrow
question_answer 175) Which of the following is the relatively most accurate method for dating of fossils?
A)
Potassium-argon method
done
clear
B)
Uranium-lead method
done
clear
C)
Electron spin resonance method
done
clear
D)
Radio-carbon method
done
clear
View Answer play_arrow
question_answer 176) During transcription holoenzyme RNA polymerase binds to a DNA sequence and the DNA assumes a saddle-like structure at that point. What is that sequence called?
A)
CAAT box
done
clear
B)
GGTT box
done
clear
C)
AAAT box
done
clear
D)
TATA box
done
clear
View Answer play_arrow
question_answer 177) Telomerase is an enzyme, which is a
A)
repetitive DNA
done
clear
B)
RNA
done
clear
C)
simple protein
done
clear
D)
ribonucleoprotein
done
clear
View Answer play_arrow
question_answer 178) Centromere is required for
A)
transcription
done
clear
B)
crossing over
done
clear
C)
cytoplasmic cleavage
done
clear
D)
movement of chromosomes towards poles
done
clear
View Answer play_arrow
question_answer 179) In Ornithine cycle, which of the following wastes are removed from the blood?
A)
Urea and urine
done
clear
B)
Ammonia and urea
done
clear
C)
Carbon dioxide and ammonia
done
clear
D)
Carbon dioxide and urea
done
clear
View Answer play_arrow
question_answer 180) Damage to thymus in a child may lead to
A)
a reduction in haemoglobin content of blood
done
clear
B)
a reduction in stem cell production
done
clear
C)
loss of antibody mediated immunity
done
clear
D)
loss of cell mediated immunity
done
clear
View Answer play_arrow
question_answer 181) Biodiversity Act of India was passed by the Parliament in the year
A)
1996
done
clear
B)
1992
done
clear
C)
2002
done
clear
D)
2000
done
clear
View Answer play_arrow
question_answer 182) During which stage in the complete oxidation of glucose, are the greatest number of ATP molecules formed from ADP?
A)
Conversion of pyruvic acid to acetyl Co-A
done
clear
B)
Electron transport chain
done
clear
C)
Glycolysis
done
clear
D)
Krebs cycle
done
clear
View Answer play_arrow
question_answer 183) A woman with normal vision, but whose father was colourblind, marries a colourblind man. Suppose that the fourth child of this couple was a boy. This boy
A)
must have normal colour vision
done
clear
B)
will be partially colourblind since he is heterozygous for the colourblind mutant allele
done
clear
C)
must be colourblind
done
clear
D)
may be colourblind or may be of normal vision
done
clear
View Answer play_arrow
question_answer 184) Which of the following is geneally used for induced mutagenesis in crop plants?
A)
Alpha particles
done
clear
B)
X-rays
done
clear
C)
UV(260nm)
done
clear
D)
Gamma rays (from cobalt 60)
done
clear
View Answer play_arrow
question_answer 185) Which one of the following depresses brain activity and produces feeling of calmness, relaxation and drowsiness?
A)
Valium
done
clear
B)
Morphine
done
clear
C)
Hashish
done
clear
D)
Amphetamines
done
clear
View Answer play_arrow
question_answer 186) Golden rice is transgenic crop of the future with the following improved trait.
A)
High lysine (essential amino acid) content
done
clear
B)
Insect resistance
done
clear
C)
High protein content
done
clear
D)
High vitamin-Acontent
done
clear
View Answer play_arrow
question_answer 187) Photosynthetic Active Radiation (PAR) has the following range of wavelengths.
A)
400 to 700 nm
done
clear
B)
450 to 950 nm
done
clear
C)
340 to 450 nm
done
clear
D)
500 to 600 nm
done
clear
View Answer play_arrow
question_answer 188) The catalytic efficiency of two different enzymes can be compared by
A)
the \[{{K}_{m}}\] value
done
clear
B)
the pH optimum value
done
clear
C)
formation of the product
done
clear
D)
molecular size of the enzyme
done
clear
View Answer play_arrow
question_answer 189) Which one of the following pairs is mismatched?
A)
Savanna - Acacia trees
done
clear
B)
Prairie - Epiphytes
done
clear
C)
Tundra - Permafrost
done
clear
D)
Coniferous forest - Evergreen tress
done
clear
View Answer play_arrow
question_answer 190) Using imprints from a plate with complete medium and carrying bacterial colonies, you can selected streptomycin resistant mutants prove that such mutations do not originate as adaptation. These imprints need to be used
A)
only on plates with streptomycin
done
clear
B)
on plates with minimal medium
done
clear
C)
only on plates without streptomycin
done
clear
D)
on plates with and without streptomycin
done
clear
View Answer play_arrow
question_answer 191) A patient is generally advised to specially consume more meat, lentils, milk and eggs in diet only, when he suffers from
A)
kwashiorkor
done
clear
B)
rickets
done
clear
C)
anaemia
done
clear
D)
scurvy
done
clear
View Answer play_arrow
question_answer 192) Epithelial cells of the intestine involved in food absorption have on their surface
A)
pinocytic vesicles
done
clear
B)
phagocytic vesicles
done
clear
C)
zymogen granules
done
clear
D)
microvilli
done
clear
View Answer play_arrow
question_answer 193) Animals have the innate ability to escape from predation. Examples for the same are given below. Select the incorrect example.
A)
Enlargement of body size by swallowing air in puffer fish
done
clear
B)
Melanism in moths
done
clear
C)
Poison fangs in snakes
done
clear
D)
Colour change in Chamaeleon
done
clear
View Answer play_arrow
question_answer 194) For retting of jute, the fermenting microbe used is
A)
Helicobactor pylori
done
clear
B)
methophilic bacteria
done
clear
C)
Streptococcus lactis
done
clear
D)
butyric acid bacteria
done
clear
View Answer play_arrow
question_answer 195) From the following statements, select the wrong one
A)
Millipedes have two pairs of appendage in each segment of the body
done
clear
B)
Prawn has two pairs of antennae
done
clear
C)
Animals belonging to phylum-Porifera are exclusively marine
done
clear
D)
Nematocysts are characteristic of phylum-Cnidaria
done
clear
View Answer play_arrow
question_answer 196) Nucleotide are building blocks of nucleic acid, nucleotide is a composite molecule formed
A)
(base-sugar-phosphate) n
done
clear
B)
base-sugar-OH
done
clear
C)
base-sugar-phosphate
done
clear
D)
sugar-phosphate
done
clear
View Answer play_arrow
question_answer 197) Which one of the following experiments suggest that simplest living organisms could not have originated spontaneously from non- living matter?
A)
Microbes did not appear in stored meat
done
clear
B)
Larvae could appear in decaying organic matter
done
clear
C)
Microbes appeared from unsterilized organic matter
done
clear
D)
Meat was not spoiled, when heated and kept sealed in a vessel
done
clear
View Answer play_arrow
question_answer 198) There exists a close association between the alga and the fungus within a lichen. The fungus
A)
fixes the atmospheric nitrogen for the alga
done
clear
B)
provides protection, anchorage and absorption for the alga
done
clear
C)
provides food for the alga
done
clear
D)
releases oxygen for the alga
done
clear
View Answer play_arrow
question_answer 199) A student wishes to study the cells structure under a light microscope having 10X eyepiece and 45X objective. He should illuminate the object by which one of the following colors of light so as to get the best possible resolution?
A)
Yellow
done
clear
B)
Green
done
clear
C)
Blue
done
clear
D)
Red
done
clear
View Answer play_arrow
question_answer 200) At what stage of the cell cycle are histone proteins synthesised in a eukaryotic cell?
A)
During entire prophase
done
clear
B)
During telophase
done
clear
C)
During S-phase
done
clear
D)
During \[{{\text{G}}_{\text{2}}}\text{-stage}\]of prophase
done
clear
View Answer play_arrow
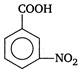
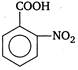
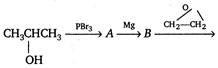 \[C\xrightarrow[{}]{{{H}_{2}}O}D\] Here, D is
\[C\xrightarrow[{}]{{{H}_{2}}O}D\] Here, D is