question_answer 1) Experimental investigations show that the intensity of solar radiation is maximum for a wavelength 480 nm in the visible region. Estimate the surface temperature of sun. (Given Wiens constant \[b=2.88\times {{10}^{-3}}mK.\])
A)
4000 K
done
clear
B)
6000 K
done
clear
C)
8000 K
done
clear
D)
\[{{10}^{6}}K\]
done
clear
View Answer play_arrow
question_answer 2) The temperature of an ideal gas is increased from 120 K to 480 K. If at 120 K, the root mean square speed of gas molecules is v, then at 480 K, it will be
A)
4v
done
clear
B)
2v
done
clear
C)
\[\frac{v}{2}\]
done
clear
D)
\[\frac{v}{4}\]
done
clear
View Answer play_arrow
question_answer 3) Two mirrors at an angle \[{{\text{ }\!\!\theta\!\!\text{ }}^{\text{o}}}\] produce 5 images of a point. The number of images produced when \[\text{ }\!\!\theta\!\!\text{ }\] is decreased to \[\theta -{{30}^{o}}\] is
A)
9
done
clear
B)
10
done
clear
C)
11
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 4) The radius of the light circle observed by a fish at a depth of 12 m is (refractive index of water = 4/3)
A)
\[36\sqrt{7}\]
done
clear
B)
\[\frac{36}{\sqrt{7}}\]
done
clear
C)
\[36\sqrt{5}\]
done
clear
D)
\[4\sqrt{5}\]
done
clear
View Answer play_arrow
question_answer 5) In Youngs double slit experiment, the fringe width is p. If the entire arrangement is placed in a liquid of refractive index n, the fringe width becomes
A)
\[n\beta \]
done
clear
B)
\[\frac{\beta }{n+1}\]
done
clear
C)
\[\frac{\beta }{n-1}\]
done
clear
D)
\[\frac{\beta }{n}\]
done
clear
View Answer play_arrow
question_answer 6) A plano-convex lens \[(f=20\,cm)\] is silvered at plane surface. Now focal length will be
A)
20 cm
done
clear
B)
40 cm
done
clear
C)
30 cm
done
clear
D)
10 cm
done
clear
View Answer play_arrow
question_answer 7) The light beams of intensities in the ratio of \[9:1\] are allowed to interfere. What will be the ratio of the intensities of maxima and minima?
A)
3 : 1
done
clear
B)
4 : 1
done
clear
C)
25 : 9
done
clear
D)
81 : 1
done
clear
View Answer play_arrow
question_answer 8) If \[{{x}_{1}}\]be the size of the magnified image and. \[{{x}_{2}}\] the size of the diminished image in Lens Displacement Method, then the size of the object is
A)
\[\sqrt{{{x}_{1}}{{x}_{2}}}\]
done
clear
B)
\[{{x}_{1}}{{x}_{2}}\]
done
clear
C)
\[x_{1}^{2}{{x}_{2}}\]
done
clear
D)
\[{{x}_{1}}x_{2}^{2}\]
done
clear
View Answer play_arrow
question_answer 9) A point charge +q is placed at the centre of a cube of side L. The electric flux emerging from the cube is
A)
\[\frac{q}{{{\varepsilon }_{0}}}\]
done
clear
B)
zero
done
clear
C)
\[\frac{6q{{L}^{2}}}{{{\varepsilon }_{0}}}\]
done
clear
D)
\[\frac{q}{6{{L}^{2}}{{\varepsilon }_{0}}}\]
done
clear
View Answer play_arrow
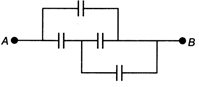
question_answer 10)
In the figure below, the capacitance of each capacitor is \[3\mu F.\] The effective capacitance I between A and B is
A)
\[\frac{3}{4}\mu F\]
done
clear
B)
\[3\,\mu F\]
done
clear
C)
\[6\,\mu F\]
done
clear
D)
\[5\,\mu F\]
done
clear
View Answer play_arrow
question_answer 11) \[n\]identical droplets are charged to V volt each. If they coalesce to form a single drop, then its potential will be
A)
\[{{n}^{2/3}}V\]
done
clear
B)
\[{{n}^{1/3}}V\]
done
clear
C)
\[nV\]
done
clear
D)
\[V/n\]
done
clear
View Answer play_arrow
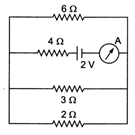
question_answer 12)
The reading of the ammeter in the following figure will be
A)
0.8 A
done
clear
B)
0.6 A
done
clear
C)
0.4 A
done
clear
D)
0.2 A
done
clear
View Answer play_arrow
question_answer 13) A wire of resistance R is elongated \[n-\]fold to make a new uniform wire. The resistance of new wire
A)
\[nR\]
done
clear
B)
\[{{n}^{2}}R\]
done
clear
C)
2nR
done
clear
D)
\[2{{n}^{2}}R\]
done
clear
View Answer play_arrow
question_answer 14) The ratio of magnetic field and magnetic moment at the centre of a current carrying circular loop is \[x.\]When both the current and radius is doubled the ratio will be
A)
\[x/8\]
done
clear
B)
\[x/4\]
done
clear
C)
\[x/2\]
done
clear
D)
\[2x\]
done
clear
View Answer play_arrow
question_answer 15) The current through a coil of self-inductance \[L=2mH\] is given by \[i={{t}^{2}}{{e}^{-t}}\]at time t. How long it will take to make the emf zero?
A)
1s
done
clear
B)
2s
done
clear
C)
3s
done
clear
D)
4 s
done
clear
View Answer play_arrow
question_answer 16) The magnetic flux through a loop of resistance \[10\,\Omega \] is given by \[(o|=5{{t}^{2}}-4t+1\,Wb).\]How much current is induced in the loop after 0.2 s?
A)
0.4 A
done
clear
B)
0.2 A
done
clear
C)
0.04 A
done
clear
D)
0.02 A
done
clear
View Answer play_arrow
question_answer 17) The decimal equivalent of the binary number \[{{(11010.101)}_{2}}\] is
A)
9.625
done
clear
B)
25.265
done
clear
C)
26.625
done
clear
D)
26.265
done
clear
View Answer play_arrow
question_answer 18) In a common emitter configuration, a transistor has \[\beta =50\] and input resistance \[\text{1}\,\text{k }\!\!\Omega\!\!\text{ }\text{.}\] If the peak value of AC input is 0.01 V, then the peak value of collector current is
A)
\[0.01\,\mu A\]
done
clear
B)
\[0.25\,\mu A\]
done
clear
C)
\[100\,\mu A\]
done
clear
D)
\[500\,\mu A\]
done
clear
View Answer play_arrow
question_answer 19) Half-life of a radioactive substance is 20 min. The time between 20% and 80% decay will be
A)
20 min
done
clear
B)
30 min
done
clear
C)
40 min
done
clear
D)
25 min
done
clear
View Answer play_arrow
question_answer 20) The energy released by the fission of one uranium atom is 200 MeV. The number of fissions per second required to produce 3.2 W of power is (Take, \[1\,eV=1.6\times {{10}^{-19}}J\])
A)
\[{{10}^{7}}\]
done
clear
B)
\[{{10}^{10}}\]
done
clear
C)
\[{{10}^{15}}\]
done
clear
D)
\[{{10}^{11}}\]
done
clear
View Answer play_arrow
question_answer 21) A body is projected with a speed \[\mu \,m/s\]at an angle \[\beta \]with the horizontal. The kinetic 3 energy at the highest point is \[\frac{3}{4}\,\text{th}\]of the 4 initial kinetic energy. The value of \[\beta \] is
A)
\[{{30}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{60}^{o}}\]
done
clear
D)
\[{{120}^{o}}\]
done
clear
View Answer play_arrow
question_answer 22) A ball is projected horizontally with a velocity of 5 m/s from the top of a building 19.6 m high. How long will the ball take to hit the ground?
A)
\[\sqrt{2}\,s\]
done
clear
B)
2s
done
clear
C)
\[\sqrt{3}\,s\]
done
clear
D)
3 s
done
clear
View Answer play_arrow
question_answer 23) A stone falls freely from rest and the total distance covered by it in the last second of its motion equals the distance covered by it in the first three seconds of its motion. The stone remains in the air for
A)
6 s
done
clear
B)
5 s
done
clear
C)
7s
done
clear
D)
4s
done
clear
View Answer play_arrow
question_answer 24)
Two blocks of 2 kg and 1 kg are in contact on a frictionless table. If a force of 3 N is applied on 2 kg block, then the force of contact between the two blocks will be
A)
zero
done
clear
B)
1 N
done
clear
C)
2N
done
clear
D)
3N
done
clear
View Answer play_arrow
question_answer 25) If momentum is increased by 20%, then kinetic energy increases by
A)
48%
done
clear
B)
44%
done
clear
C)
40%
done
clear
D)
36%
done
clear
View Answer play_arrow
question_answer 26) A boy of mass 40 kg is climbing a vertical pole at a constant speed. If the coefficient of friction between his palms and the pole is 0.8 and \[g=10\,m/{{s}^{2}},\]the horizontal force that he is applying on the pole is
A)
300 N
done
clear
B)
400 N
done
clear
C)
500 N
done
clear
D)
600 N
done
clear
View Answer play_arrow
question_answer 27) The value of \[\lambda \] for which the two vectors \[\vec{a}=5\hat{i}+\lambda \hat{j}+\hat{k}\]and \[\vec{b}=\hat{i}-2\hat{j}+\hat{k}\] are perpendicular to each other is
A)
2
done
clear
B)
-2
done
clear
C)
3
done
clear
D)
-3
done
clear
View Answer play_arrow
question_answer 28) If \[\vec{a}+\vec{b}+\vec{c}\]and \[a+b=c,\]then the angle included between \[\vec{a}\]and \[\vec{b}\]is
A)
\[{{90}^{o}}\]
done
clear
B)
\[{{180}^{o}}\]
done
clear
C)
\[{{120}^{o}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 29) The height vertically above the earths surface at which the acceleration due to gravity becomes 1% of its value at the surface is (R is the radius of the earth)
A)
8R
done
clear
B)
9R
done
clear
C)
10R
done
clear
D)
20R
done
clear
View Answer play_arrow
question_answer 30) The change in the gravitational potential energy when a body of mass m is raised to a height nR above the surface of the Earth is (Here R is the radius of the earth)
A)
\[\left( \frac{n}{n+1} \right)mgR\]
done
clear
B)
\[\left( \frac{n}{n-1} \right)mgR\]
done
clear
C)
\[nmgR\]
done
clear
D)
\[\frac{mgR}{n}\]
done
clear
View Answer play_arrow
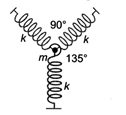
question_answer 31)
A particle of mass m is attached to three identical massless springs of spring constant k as shown in the figure. The time period of vertical oscillation of the particle is
A)
\[2\pi \sqrt{\frac{m}{k}}\]
done
clear
B)
\[2\pi \sqrt{\frac{m}{2k}}\]
done
clear
C)
\[2\pi \sqrt{\frac{m}{3k}}\]
done
clear
D)
\[\pi \sqrt{\frac{m}{k}}\]
done
clear
View Answer play_arrow
question_answer 32) A spring of force constant k is cut into three equal parts. The force constant of each part would be
A)
\[\frac{k}{3}\]
done
clear
B)
\[3k\]
done
clear
C)
\[k\]
done
clear
D)
\[2k\]
done
clear
View Answer play_arrow
question_answer 33) A body floats in water with 40% of its volume outside water. When the same body floats in oil, 60% of its volume remains outside oil. The relative density of the oil is
A)
0.9
done
clear
B)
1.2
done
clear
C)
1.5
done
clear
D)
1.8
done
clear
View Answer play_arrow
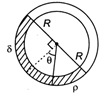
question_answer 34)
A uniform long tube is bent into a circle of radius R und it lies in vertical plane. Two liquids of same volume but densities \[\rho \]and \[\delta \] fill half the tube. The angle \[\theta \] is
A)
\[{{\tan }^{-1}}\left( \frac{\rho -\delta }{\rho +\delta } \right)\]
done
clear
B)
\[{{\tan }^{-1}}\left( \frac{\rho }{\delta } \right)\]
done
clear
C)
\[{{\tan }^{-1}}\left( \frac{\delta }{\rho } \right)\]
done
clear
D)
\[{{\tan }^{-1}}\left( \frac{\rho +\delta }{\rho -\delta } \right)\]
done
clear
View Answer play_arrow
question_answer 35) Two solid spheres of same metal but of mass M and 8M fall simultaneously on a viscous liquid and their terminal velocities are v and nv, then value of n is
A)
16
done
clear
B)
8
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 36) A particle is executing linear simple harmonic motion of amplitude A. At what displacement is the energy of the particle half potential and half kinetic?
A)
\[\frac{A}{4}\]
done
clear
B)
\[\frac{A}{2}\]
done
clear
C)
\[\frac{A}{\sqrt{2}}\]
done
clear
D)
\[\frac{A}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 37) The equation of a progressive wave is \[y=4\sin (4\pi t-0.04x+\pi /3)\]where \[x\]is in metre and t is in second. The velocity of the wave is
A)
\[100\pi m/s\]
done
clear
B)
\[50\pi \,m/s\]
done
clear
C)
\[25\pi \,m/s\]
done
clear
D)
\[\pi \,m/s\]
done
clear
View Answer play_arrow
question_answer 38) A longitudinal wave is represented by \[x={{x}_{0}}\sin 2\pi (nt-x/\lambda ).\]The maximum particle velocity will be four times the wave velocity if
A)
\[\lambda =\frac{\pi {{x}_{0}}}{4}\]
done
clear
B)
\[\lambda =2\pi {{x}_{0}}\]
done
clear
C)
\[\lambda =\frac{\pi {{x}_{0}}}{2}\]
done
clear
D)
\[\lambda =4\pi {{x}_{0}}\]
done
clear
View Answer play_arrow
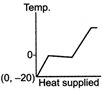
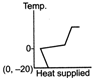
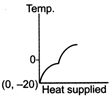
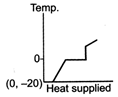
question_answer 39) A block of ice at temperature \[-\text{20}{{\,}^{\text{o}}}\text{C}\]is slowly heated and converted to steam at \[\text{100}{{\,}^{\text{o}}}\text{C}\] Which of the following diagram is most appropriate?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 40) Two black bodies at temperatures \[\text{327}{{\,}^{\text{o}}}\text{C}\]and \[\text{427}{{\,}^{\text{o}}}\text{C}\]are kept in an evacuated chamber at \[\text{27}{{\,}^{\text{o}}}\text{C}\] The ratio of their rates of loss of heat are
A)
\[\frac{6}{7}\]
done
clear
B)
\[{{\left( \frac{6}{7} \right)}^{2}}\]
done
clear
C)
\[{{\left( \frac{6}{7} \right)}^{3}}\]
done
clear
D)
\[\frac{243}{464}\]
done
clear
View Answer play_arrow
question_answer 41) At identical temperature and pressure, the rate of diffusion of hydrogen gas is \[3\sqrt{3}\] times that of a hydrocarbon having molecular formula \[{{C}_{n}}{{H}_{2n-2}}.\]What is the value of n?
A)
1
done
clear
B)
4
done
clear
C)
3
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 42)
Dipole moment of
A)
1.5 D
done
clear
B)
2.25 D
done
clear
C)
1 D
done
clear
D)
3 D
done
clear
View Answer play_arrow
question_answer 43) Which of the following thermodynamic relation is correct?
A)
\[dG=Vdp-SdT\]
done
clear
B)
\[dE=pdV+TdS\]
done
clear
C)
\[dH=-Vdp+TdS\]
done
clear
D)
\[dG=Vdp+SdT\]
done
clear
View Answer play_arrow
question_answer 44) In the hydrolysis of an organic chloride in presence of large excess of water, \[RCl+{{H}_{2}}O\to ROH+HCl\]
A)
molecularity and order of reaction both are 2
done
clear
B)
molecularity is 2 but order of reaction is 1
done
clear
C)
molecularity is 1 but order of reaction is 2
done
clear
D)
molecularity is 1 and order of reaction is also 1
done
clear
View Answer play_arrow
question_answer 45) The potential of a hydrogen electrode at pH=10 is
A)
0.59 V
done
clear
B)
0.00 V
done
clear
C)
-0.59 V
done
clear
D)
-0.059
done
clear
View Answer play_arrow
question_answer 46) Calculate \[{{K}_{c}}\]for the reversible process given below if \[{{K}_{p}}=167\]and \[T=800{{\,}^{o}}C\] \[CaC{{O}_{3}}(s)CaO(s)+C{{O}_{2}}(g)\]
A)
1.95
done
clear
B)
1.85
done
clear
C)
1.89
done
clear
D)
1.60
done
clear
View Answer play_arrow
question_answer 47) For a reversible chemical reaction where the forward process is exothermic, which of the following statements is correct?
A)
The backward reaction has higher activation energy than the forward reaction
done
clear
B)
The backward and the forward processes have the same activation energy
done
clear
C)
The backward reaction has lower activation energy
done
clear
D)
No activation energy is required at all since energy is liberated in the process
done
clear
View Answer play_arrow
question_answer 48) In Sommerfelds modification of Bohrs theory, the trajectory of an electron in a hydrogen atom is
A)
a perfect ellipse
done
clear
B)
a closed ellipse-like curve, narrower at the perihelion position and flatter at the aphelion position
done
clear
C)
a closed loop on spherical surface
done
clear
D)
a rosette
done
clear
View Answer play_arrow
question_answer 49) In the reaction of sodium thiosulphate with \[{{I}_{2}}\]in aqueous medium, the equivalent weight of sodium thiosulphate is equal to
A)
molar mass of sodium thiosulphate
done
clear
B)
the average of molar masses of \[\text{N}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\] and 12
done
clear
C)
half the molar mass of sodium thiosulphate
done
clear
D)
molar mass of sodium thiosulphate \[\times \,\text{2}\]
done
clear
View Answer play_arrow
question_answer 50) 0.1 M HCl and \[\text{0}\text{.1 M }{{\text{H}}_{\text{2}}}\text{SO4}\]each of volume 2 mL are mixed and the volume is made upto 6 mL by adding 2 mL of 0.01 N NaCI solution. The pH of the resulting mixture is
A)
1.17
done
clear
B)
1.0
done
clear
C)
0.3
done
clear
D)
log 2-log 3
done
clear
View Answer play_arrow
question_answer 51) The molarity of a NaOH solution by dissolving 4 g of it in 250 mL water is
A)
0.4 M
done
clear
B)
0.8 M
done
clear
C)
0.2 M
done
clear
D)
0.1 M
done
clear
View Answer play_arrow
question_answer 52) If a species has 16 protons, 18 electrons and 16 neutrons, find the species and its charge.
A)
\[{{S}^{-1}}\]
done
clear
B)
\[S{{i}^{2-}}\]
done
clear
C)
\[{{P}^{3-}}\]
done
clear
D)
\[{{S}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 53) In Periodic Table, the basic character of oxides
A)
increases from left to right and decreases from top to bottom
done
clear
B)
decreases from right to left and increases from top to bottom
done
clear
C)
decreases from left to right and increases from top to bottom
done
clear
D)
decreases from left to right and increases from bottom to top
done
clear
View Answer play_arrow
question_answer 54) Which one of the following contains P-O-P bond?
A)
Hypophosphorus acid
done
clear
B)
Phosphorus acid
done
clear
C)
Pyrophosphoric acid
done
clear
D)
Orthophosphoric acid
done
clear
View Answer play_arrow
question_answer 55) Which of the following orders regarding ionisation energy is correct?
A)
N > O > F
done
clear
B)
N < O < F
done
clear
C)
N > O < F
done
clear
D)
N < O > F
done
clear
View Answer play_arrow
question_answer 56) Which of the following statements regarding ozone is not correct?
A)
The ozone molecule is angular in shape
done
clear
B)
The ozone is a resonance hybrid of two structures
done
clear
C)
The oxygen-oxygen bond length in ozone is identical with that of molecular oxygen
done
clear
D)
Ozone is used as germicide and disinfectant for the purification of air
done
clear
View Answer play_arrow
question_answer 57) \[{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}\]is the anhydride of
A)
\[{{H}_{3}}P{{O}_{2}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
D)
\[{{H}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
View Answer play_arrow
question_answer 58) Which of the following metals has the largest abundance in the earths crust?
A)
Aluminium
done
clear
B)
Calcium
done
clear
C)
Magnesium
done
clear
D)
Sodium
done
clear
View Answer play_arrow
question_answer 59) Which of the following orbitals will have zero probability of finding the electron in the yz plane?
A)
\[{{p}_{x}}\]
done
clear
B)
\[{{p}_{y}}\]
done
clear
C)
\[{{p}_{z}}\]
done
clear
D)
\[{{d}_{yz}}\]
done
clear
View Answer play_arrow
question_answer 60) What type of orbital hybridisation is considered on P in \[PC{{l}_{5}}\]?
A)
\[s{{p}^{3}}d\]
done
clear
B)
\[ds{{p}^{3}}\]
done
clear
C)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
D)
\[{{d}^{2}}s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 61) For which element the inertness of the electron pair will not be observed?
A)
Sn
done
clear
B)
Fe
done
clear
C)
Pb
done
clear
D)
In
done
clear
View Answer play_arrow
question_answer 62) In which of the following molecules is hydrogen bridge bond present?
A)
Water
done
clear
B)
Inorganic benzene
done
clear
C)
Diborane
done
clear
D)
Methanol
done
clear
View Answer play_arrow
question_answer 63) When a manganous salt is fused with a mixture of \[KN{{O}_{3}}\]and solid \[\text{NaOH,}\] the oxidation number of Mn changes from +2 to
A)
+4
done
clear
B)
+3
done
clear
C)
+6
done
clear
D)
+7
done
clear
View Answer play_arrow
question_answer 64) In haemoglobin, the metal ion present is
A)
\[F{{e}^{2+}}\]
done
clear
B)
\[Z{{n}^{2+}}\]
done
clear
C)
\[C{{o}^{2+}}\]
done
clear
D)
\[C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 65) Ortho-and para-hydrogens have
A)
identical chemical properties but different physical properties
done
clear
B)
identical physical and chemical properties
done
clear
C)
identical physical properties but different chemical properties
done
clear
D)
different physical and chemical properties
done
clear
View Answer play_arrow
question_answer 66) The bond order of CO molecule is
A)
2
done
clear
B)
2.5
done
clear
C)
3
done
clear
D)
3.5
done
clear
View Answer play_arrow
question_answer 67) Vitamin C is
A)
citric acid
done
clear
B)
lactic acid
done
clear
C)
paracetamol
done
clear
D)
ascorbic acid
done
clear
View Answer play_arrow
question_answer 68) On mixing alkane with chlorine and irradiating with ultraviolet light, it forms only one monochloroalkane. The alkane is
A)
propane
done
clear
B)
pentane
done
clear
C)
150-pentane
done
clear
D)
neo-pentane
done
clear
View Answer play_arrow
question_answer 69) Keto-enol tautomerism is not observed in
A)
\[{{C}_{6}}{{H}_{5}}CO{{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}COCH=C{{H}_{2}}\]
done
clear
C)
\[~{{C}_{6}}{{H}_{5}}COC{{H}_{2}}COC{{H}_{3}}\]
done
clear
D)
\[~C{{H}_{3}}COC{{H}_{2}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 70) What is obtained when nitrobenzene is treated sequentially with (i) \[N{{H}_{4}}Cl/Zn\]dust and (ii) \[{{H}_{2}}S{{O}_{4}}/N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\]
A)
meta-chloronitrobenzene
done
clear
B)
para-chloronitrobenzene
done
clear
C)
Nitrosobenzene
done
clear
D)
Benzene
done
clear
View Answer play_arrow
question_answer 71) Boiling water reacts with \[{{C}_{6}}{{H}_{5}}N_{2}^{+}C{{l}^{-}}\]to give
A)
aniline
done
clear
B)
benzylamine
done
clear
C)
phenol
done
clear
D)
benzaldehyde
done
clear
View Answer play_arrow
question_answer 72) Aspirin is
A)
acetyl salicylic acid
done
clear
B)
benzoyl salicylic acid
done
clear
C)
chloro benzoic acid
done
clear
D)
anthranilic acid
done
clear
View Answer play_arrow
question_answer 73) \[X\xrightarrow{PC{{l}_{5}}}{{C}_{2}}{{H}_{5}}Cl,Y\xrightarrow{PC{{l}_{5}}}C{{H}_{3}}COCl\] X and Y are
A)
\[{{({{C}_{2}}{{H}_{5}})}_{2}}O\]and \[C{{H}_{3}}C{{O}_{2}}H\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}I\]and \[{{C}_{2}}{{H}_{5}}CHO\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}OH\]and \[C{{H}_{3}}C{{O}_{2}}H\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH\]and \[{{C}_{2}}{{H}_{5}}CHO\]
done
clear
View Answer play_arrow
question_answer 74) Which of the following compounds shows evidence of the strongest hydrogen bonding?
A)
Propan-1-ol
done
clear
B)
Propan-2-ol
done
clear
C)
Propan-1, 2-diol
done
clear
D)
Propan-1, 2, 3-triol
done
clear
View Answer play_arrow
question_answer 75) When AgCl is treated with KCN,
A)
Ag is precipitated
done
clear
B)
a complex ion is formed
done
clear
C)
double decomposition takes place
done
clear
D)
no reaction takes place
done
clear
View Answer play_arrow
question_answer 76) Which one of the following produced when acetone is saturated with HCl gas?
A)
Acetone alcohol
done
clear
B)
Phorone
done
clear
C)
Mesityl oxide
done
clear
D)
Benzene
done
clear
View Answer play_arrow
question_answer 77) Which one of the following is an example of co-polymer?
A)
Buna-S
done
clear
B)
Teflon
done
clear
C)
PVC
done
clear
D)
Polypropylene
done
clear
View Answer play_arrow
question_answer 78) Identify [A] and [B] in the following\[_{89}^{227}Ac\xrightarrow{-\beta }[A]\xrightarrow{-\alpha }[B]\xrightarrow{-\alpha }Rn\]
A)
Po, Rn
done
clear
B)
Th, Po
done
clear
C)
Ra, Th
done
clear
D)
Th, Ra
done
clear
View Answer play_arrow
question_answer 79) A weak acid of dissociation constant \[{{10}^{-5}}\] is being titrated with aqueous \[NaOH\] solution. The pH at the point of one-third neutralisation of the acid will be
A)
\[5+\log 2-\log 3\]
done
clear
B)
\[5-\log 2\]
done
clear
C)
\[5-\log 3\]
done
clear
D)
\[5-\log 6\]
done
clear
View Answer play_arrow
question_answer 80) Radioactivity of a sample (Z = 22) decreases 90% after 10 yr. What will be the half-life of the sample?
A)
5yr
done
clear
B)
2yr
done
clear
C)
3yr
done
clear
D)
10 yr
done
clear
View Answer play_arrow
question_answer 81) How many variable segments are present in the basic structure of antibody molecule?
A)
One
done
clear
B)
Two
done
clear
C)
Three
done
clear
D)
Four
done
clear
View Answer play_arrow
question_answer 82) Which one is diaminodicarboxylic amino add?
A)
Cystine
done
clear
B)
Lysine
done
clear
C)
Cysteine
done
clear
D)
Aspartic acid
done
clear
View Answer play_arrow
question_answer 83) Which one is the cofactor of carbonic anhydrase?
A)
Iron (Fe)
done
clear
B)
Zinc (Zn)
done
clear
C)
Copper (Cu)
done
clear
D)
Magnesium (Mg)
done
clear
View Answer play_arrow
question_answer 84) Vitamin - D is produced in human body in
A)
muscles
done
clear
B)
nerves
done
clear
C)
skin
done
clear
D)
bone marrow
done
clear
View Answer play_arrow
question_answer 85) Bacteriophages kill
A)
fungi
done
clear
B)
parasites
done
clear
C)
bacteria
done
clear
D)
viruses
done
clear
View Answer play_arrow
question_answer 86) What is mitoplast?
A)
Membraneless mitochondria
done
clear
B)
Another name of mitochondria
done
clear
C)
Mitochondria without outer membrane
done
clear
D)
Mitochondria without inner membrane
done
clear
View Answer play_arrow
question_answer 87) Transposons are
A)
house- keeping genes
done
clear
B)
jumping genes
done
clear
C)
transporting genes
done
clear
D)
stationary genes
done
clear
View Answer play_arrow
question_answer 88) Which of the following is not a conjugated protein?
A)
Peptone
done
clear
B)
Phosphoprotein
done
clear
C)
Lipoprotein
done
clear
D)
Chromoprotein
done
clear
View Answer play_arrow
question_answer 89) The outer covering of cartilage is called
A)
peritonium
done
clear
B)
periosteum
done
clear
C)
endosteum
done
clear
D)
perichondrium
done
clear
View Answer play_arrow
question_answer 90) The blood does not clot inside the body because of
A)
oxygenation of blood
done
clear
B)
movement of blood
done
clear
C)
heparin in blood
done
clear
D)
absence of fibrinogen in blood
done
clear
View Answer play_arrow
question_answer 91) Red cell count is carried out by
A)
haemocytometer
done
clear
B)
haemoglobinometer
done
clear
C)
sphygmomanometer
done
clear
D)
electrocardiogram
done
clear
View Answer play_arrow
question_answer 92) Rh factor can produce disease
A)
AIDS
done
clear
B)
Turners syndrome
done
clear
C)
Erythroblastosis foetalis
done
clear
D)
Sickle- cell anaemia
done
clear
View Answer play_arrow
question_answer 93) Name the hormone that stimulates the secretion of gastric juice.
A)
Renin
done
clear
B)
Enterokinase
done
clear
C)
Enterogastrone
done
clear
D)
Gastrin
done
clear
View Answer play_arrow
question_answer 94) Bile salts act as activator of which enzyme?
A)
Pepsinogen
done
clear
B)
Trypsinogen
done
clear
C)
Lipase
done
clear
D)
Pancreatic amylase
done
clear
View Answer play_arrow
question_answer 95) Heparin is produced by
A)
kidney cells
done
clear
B)
blood cells
done
clear
C)
bone marrow
done
clear
D)
liver cells
done
clear
View Answer play_arrow
question_answer 96) Which of the following cells produce HCl?
A)
\[\beta \] - cell
done
clear
B)
\[\alpha \] - cell
done
clear
C)
Oxyntic cell
done
clear
D)
Chief cell
done
clear
View Answer play_arrow
question_answer 97) Which ribs show bucket - handle type of movement?
A)
Rib no. 1-2
done
clear
B)
Rib no. 3-5
done
clear
C)
Rib no. 6-10
done
clear
D)
Rib no. 11-12
done
clear
View Answer play_arrow
question_answer 98) In which of the following subjects, the dead space is highest?
A)
Old man
done
clear
B)
Old woman
done
clear
C)
Young man
done
clear
D)
Young woman
done
clear
View Answer play_arrow
question_answer 99) Which one has the thickest wall?
A)
Right auricle
done
clear
B)
Right ventricle
done
clear
C)
Left auricle
done
clear
D)
Left ventricle
done
clear
View Answer play_arrow
question_answer 100) The cardiac cycle in normal subject is about
A)
0.5 s
done
clear
B)
0.8 s
done
clear
C)
1.0s
done
clear
D)
1.2s
done
clear
View Answer play_arrow
question_answer 101) What is glycosuria?
A)
Low amount of sugar in urine
done
clear
B)
Low amount of fat in urine
done
clear
C)
Average amount of carbohydrate in urine
done
clear
D)
High amount of sugar in urine
done
clear
View Answer play_arrow
question_answer 102) Volume of urine is regulated by
A)
aldosterone
done
clear
B)
aldosterone and testosterone
done
clear
C)
ADH
done
clear
D)
aldosterone and ADH
done
clear
View Answer play_arrow
question_answer 103) Skin is an accessory organ of respiration in
A)
human
done
clear
B)
frog\
done
clear
C)
rabbit
done
clear
D)
lizard
done
clear
View Answer play_arrow
question_answer 104) Name the condition when the concentration of ketone body increases in urine.
A)
Acromegaly
done
clear
B)
Diabetes mellitus
done
clear
C)
Diabetes insipidus
done
clear
D)
Cushings disease
done
clear
View Answer play_arrow
question_answer 105) Hormone responsible for the secretion of milk after parturition is
A)
ICSH
done
clear
B)
Prolactin
done
clear
C)
ACTH
done
clear
D)
LH
done
clear
View Answer play_arrow
question_answer 106) Endemic goitre is a state of
A)
increased thyroid function
done
clear
B)
normal thyroid function
done
clear
C)
decreased thyroid function
done
clear
D)
moderate thyroid function
done
clear
View Answer play_arrow
question_answer 107) Islets of Langerhans are found as
A)
anterior pituitary
done
clear
B)
kidney cortex
done
clear
C)
spleen
done
clear
D)
endocrine pancreas
done
clear
View Answer play_arrow
question_answer 108) Which of the following is the function of adrenalin?
A)
Helps in gastric juice secretion
done
clear
B)
Increases heart rate and blood pressure
done
clear
C)
Increases blood calcium
done
clear
D)
Helps in milk secretion
done
clear
View Answer play_arrow
question_answer 109) Which of the following is not related to the autonomic nervous system?
A)
Peristalsis
done
clear
B)
Digestion
done
clear
C)
Excretion
done
clear
D)
Memory and learning
done
clear
View Answer play_arrow
question_answer 110) Comprehension of spoken and written words take place in the region of
A)
association area
done
clear
B)
motor area
done
clear
C)
Wernickes area
done
clear
D)
Brocas area
done
clear
View Answer play_arrow
question_answer 111) Which one of the following cranial nerves is carrying the nerve fibres originating from the Edinger- Westphal nucleus?
A)
Oculomotor
done
clear
B)
Trochlear
done
clear
C)
Abducens
done
clear
D)
Vagus
done
clear
View Answer play_arrow
question_answer 112) How many laminae are present in the grey matter of spinal cord?
A)
Four
done
clear
B)
Six
done
clear
C)
Eight
done
clear
D)
Ten
done
clear
View Answer play_arrow
question_answer 113) Colour blindness is due to defect in
A)
cones
done
clear
B)
rods
done
clear
C)
rods and cones
done
clear
D)
rhodopsin
done
clear
View Answer play_arrow
question_answer 114) MRI is not allowed in the following conditions except one. Identify the exception.
A)
Presence of pacemaker in the body
done
clear
B)
Pregnant women
done
clear
C)
Person suffering from stroke
done
clear
D)
Presence of metallic plate in the body for treatment of broken bones
done
clear
View Answer play_arrow
question_answer 115) Which of the following diseases is related to cadmium pollution?
A)
Minamata
done
clear
B)
Pneumoconiosis
done
clear
C)
Anaemia
done
clear
D)
Itai- itai
done
clear
View Answer play_arrow
question_answer 116) First genetically modified plant commercially released in India is
A)
golden rice
done
clear
B)
slow ripening tomato
done
clear
C)
Bt brinjal
done
clear
D)
Bt cotton
done
clear
View Answer play_arrow
question_answer 117) Quiescent centre is found in plants at
A)
root tip
done
clear
B)
cambium
done
clear
C)
shoot tip
done
clear
D)
leaf tip
done
clear
View Answer play_arrow
question_answer 118) In a DNA molecule, distance between two bases is
A)
2nm/20 \[Ao\]
done
clear
B)
0.2nm/2 \[Ao\]
done
clear
C)
3.4nm/34 \[Ao\]
done
clear
D)
0.34nm/3.4 \[Ao\]
done
clear
View Answer play_arrow
question_answer 119) Exine of pollen grain is made up of
A)
pectocellulose
done
clear
B)
lignocellulose
done
clear
C)
sporopollenin
done
clear
D)
pollen kit
done
clear
View Answer play_arrow
question_answer 120) When the cell is fully turgid, its
A)
DPD = OP
done
clear
B)
DPD = zero
done
clear
C)
WP = TP
done
clear
D)
OP = zero
done
clear
View Answer play_arrow
question_answer 121) Which one is true for ATP?
A)
ATP is a prosthetic part of an enzyme
done
clear
B)
ATP is an enzyme
done
clear
C)
ATP is the organic ions of enzyme
done
clear
D)
ATP is a coenzyme
done
clear
View Answer play_arrow
question_answer 122) Root cells of wheat has 2n = 42 chromosomes. Which one of the following is the basic chromosome number of wheat?
A)
42
done
clear
B)
21
done
clear
C)
7
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 123) Purines possess nitrogen at
A)
1, 2, 4 and 6 position
done
clear
B)
1, 3, 5 and 7 position
done
clear
C)
1, 3, 7 and 9 position
done
clear
D)
1, 2, 6 and 8 position
done
clear
View Answer play_arrow
question_answer 124) Thylakoids occur inside
A)
mitochondria
done
clear
B)
chloroplast
done
clear
C)
Golgi apparatus
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 125) Micropropagation is a technique for production of
A)
true to type plants
done
clear
B)
haploid plant
done
clear
C)
somatic hybrids
done
clear
D)
somaclonal plants
done
clear
View Answer play_arrow
question_answer 126) Test cross is a cross between
A)
hybrid and dominant parent
done
clear
B)
hybrid and recessive parent
done
clear
C)
two hybrid parents
done
clear
D)
two distantly related species
done
clear
View Answer play_arrow
question_answer 127) Mitochondria are semiautonomous as they possess
A)
DNA
done
clear
B)
DNA and RNA
done
clear
C)
DNA, RNA and ribosomes
done
clear
D)
protein
done
clear
View Answer play_arrow
question_answer 128) Chitin is a
A)
polysaccharide
done
clear
B)
nitrogenous polysaccharide
done
clear
C)
lipoprotein
done
clear
D)
protein
done
clear
View Answer play_arrow
question_answer 129) Balbiani rings are the sites of
A)
DNA replication
done
clear
B)
RNA and protein synthesis
done
clear
C)
synthesis of lipids
done
clear
D)
synthesis of polysaccharides
done
clear
View Answer play_arrow
question_answer 130) Which of the cell organelle lacks membrane
A)
Mesosome
done
clear
B)
Mitochondria
done
clear
C)
Ribosome
done
clear
D)
Liposome
done
clear
View Answer play_arrow
question_answer 131) Interfascicular cambium is a
A)
primary meristematic tissue
done
clear
B)
primordial meristem
done
clear
C)
type of protoderm
done
clear
D)
secondary meristematic tissue
done
clear
View Answer play_arrow
question_answer 132) Cotton fibre is basically a type of
A)
trichome
done
clear
B)
scale
done
clear
C)
dried seed coat
done
clear
D)
non glandular hair
done
clear
View Answer play_arrow
question_answer 133) Chloroplast dimorphism is a characteristic feature of
A)
plants with Calvin cycle
done
clear
B)
\[{{C}_{4}}-\]plants
done
clear
C)
all plants
done
clear
D)
only in algae
done
clear
View Answer play_arrow
question_answer 134) In which type of reactions related to plant photosynthesis peroxisomes are involved?
A)
Glycolate cycle
done
clear
B)
Calvin cycle
done
clear
C)
Bacterial photosynthesis
done
clear
D)
Glyoxylate cycle
done
clear
View Answer play_arrow
question_answer 135) The term alpha diversity refers to
A)
genetic diversity
done
clear
B)
community and ecosystem diversity
done
clear
C)
species diversity
done
clear
D)
diversity among the plants
done
clear
View Answer play_arrow
question_answer 136) Percentage composition of fibrin and serin in silk is
A)
50 : 40
done
clear
B)
80 : 20
done
clear
C)
30 : 70
done
clear
D)
40 : 60
done
clear
View Answer play_arrow
question_answer 137) Which one of the following is used as biological insecticide?
A)
Tiger beetle
done
clear
B)
Caterpillar
done
clear
C)
Silkmoth
done
clear
D)
Mazra poka
done
clear
View Answer play_arrow
question_answer 138) Which one of the following diseases is spread by housefly?
A)
Dengue fever
done
clear
B)
Encephalitis
done
clear
C)
Filariasis
done
clear
D)
Typhoid
done
clear
View Answer play_arrow
question_answer 139) Water vascular system is found in
A)
sea-anemone
done
clear
B)
sea-pen
done
clear
C)
sea-cucumber
done
clear
D)
sea-horse
done
clear
View Answer play_arrow
question_answer 140) Nutrient enrichment of a lake will cause
A)
eutrophication
done
clear
B)
stratification
done
clear
C)
biomagnification
done
clear
D)
bioaccumulation
done
clear
View Answer play_arrow
question_answer 141) Lichens are described as indicator of
A)
air pollution
done
clear
B)
water pollution
done
clear
C)
soil pollution
done
clear
D)
agriculture productivity
done
clear
View Answer play_arrow
question_answer 142) Most abundant mineral of animal body is
A)
iron (Fe)
done
clear
B)
sodium (Na)
done
clear
C)
potassium (K)
done
clear
D)
calcium (Ca)
done
clear
View Answer play_arrow
question_answer 143) Retrogressive metamorphosis occurs in
A)
Hemichordata
done
clear
B)
Cephalochordata
done
clear
C)
Urochordata
done
clear
D)
Vertebrata
done
clear
View Answer play_arrow
question_answer 144) Organ of Jacobson helps in
A)
touch
done
clear
B)
vision
done
clear
C)
smell
done
clear
D)
hear
done
clear
View Answer play_arrow
question_answer 145) Cysticercus stage is formed in
A)
Taenia
done
clear
B)
Plasmodium
done
clear
C)
Leishmania
done
clear
D)
Wuchereria
done
clear
View Answer play_arrow
question_answer 146) Which one of the following viruses contains both DNA and RNA?
A)
Cyanophage
done
clear
B)
Herpes virus
done
clear
C)
Leuko virus
done
clear
D)
Polio virus
done
clear
View Answer play_arrow
question_answer 147) The hormone responsible for Fight -and Flight response is
A)
adrenalin
done
clear
B)
thyroxin
done
clear
C)
ADH
done
clear
D)
oxytocin
done
clear
View Answer play_arrow
question_answer 148) Tuberculosis is caused by
A)
Mycobacterium sp.
done
clear
B)
Aspergiilus sp.
done
clear
C)
Clostridium sp.
done
clear
D)
Vibrio sp.
done
clear
View Answer play_arrow
question_answer 149) Which of the following is a catadromous fish?
A)
Hilsa sp.
done
clear
B)
Mystus sp.
done
clear
C)
Anguilla sp.
done
clear
D)
Channa sp.
done
clear
View Answer play_arrow
question_answer 150) Which of the following animal belongs to class?Crustacea?
A)
Cockroach
done
clear
B)
Cyclops
done
clear
C)
Grasshopper
done
clear
D)
Mosquito
done
clear
View Answer play_arrow
question_answer 151) Radula is found in
A)
Pila sp.
done
clear
B)
Chiton sp.
done
clear
C)
Lamellidens sp.
done
clear
D)
Pinctada sp.
done
clear
View Answer play_arrow
question_answer 152) The scientific name of Java man is
A)
Homo habilis
done
clear
B)
Homo sapiens neandarthalensis
done
clear
C)
Homo erectus erectus
done
clear
D)
Australopithecus bisei
done
clear
View Answer play_arrow
question_answer 153) Which phase comes in between the \[{{G}_{1}}\]and \[{{G}_{2}}\] phases of cell cycle?
A)
M - phase
done
clear
B)
Go - phase
done
clear
C)
S - phase
done
clear
D)
Interphase
done
clear
View Answer play_arrow
question_answer 154) How many effective codons are there for the synthesis of twenty amino acids?
A)
64
done
clear
B)
32
done
clear
C)
60
done
clear
D)
61
done
clear
View Answer play_arrow
question_answer 155) Which of the following condition is called monosomic?
A)
2n + 1
done
clear
B)
2n + 2
done
clear
C)
n + 1
done
clear
D)
2n - 1
done
clear
View Answer play_arrow
question_answer 156) Chromosome is made up of
A)
DNA and pectin
done
clear
B)
RNA and DNA
done
clear
C)
DNA and histone
done
clear
D)
only histone
done
clear
View Answer play_arrow
question_answer 157) Cell division cannot be stopped in which phase of the cell cycle?
A)
\[{{G}_{1}}-\] phase
done
clear
B)
\[{{G}_{2}}-\] phase
done
clear
C)
S - phase
done
clear
D)
Prophase
done
clear
View Answer play_arrow
question_answer 158) Which of the following is structural subunit of DNA?
A)
Protein
done
clear
B)
Carbohydrate
done
clear
C)
RNA
done
clear
D)
Nucleotide
done
clear
View Answer play_arrow
question_answer 159) Cell theory is not applicable for
A)
bacteria
done
clear
B)
fungus
done
clear
C)
algae
done
clear
D)
virus
done
clear
View Answer play_arrow
question_answer 160) The difference between systolic and diastolic pressure in human is
A)
120mmHg
done
clear
B)
80 mm Hg
done
clear
C)
40 mm Hg
done
clear
D)
200 mm Hg
done
clear
View Answer play_arrow

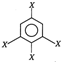 is
is
