Atoms and Molecules
Synopsis
- The simplest form of matter which can neither be split into nor built up from two or more dissimilar substances is called an element.
- An atom of an element is denoted by the symbol of that element.
- An atom of an element also denotes the atomic mass possessed by that element.
- Each atom of an element is characterised by its atomic number and mass number.
- Atomic weight of an element in grams is called gram atomic weight or gram atom.
- The smallest part of a substance that can exist independently is called a molecule.
- An aggregation of two or more atoms of the same or different elements is called a molecule.
- Molecules are characterised by their molecular weights.
- Law of Conservation of Mass: In a chemical reaction, the sum of the masses of the reactants and the products remains constant.
- Law of Definite Proportions: A chemical compound always consists of the same elements which combine in the same fixed ratio by weight.
- Law of Multiple Proportions: When two elements combine to form two or more compounds, the different weights of one element which combine with a fixed weight of the other bear a simple integral ratio.
- Molecular weight of a compound expressed in grams is called gram molecular weight or gram molecule.
- A mole is the quantity of substance that contains the same number of particles as are present in 12 g of carbon (or) mole is the collection of Avogadro's number of particles.
- Avogadro's number is \[6.023\times 1023\]. It is denoted by N.
- Empirical formula is the ratio of atoms of various elements in a compound and it is derived from percentage composition.
- Molecular formula represents the actual number of atoms of each element in a compound.
- Molecular formula = (empirical formula) \[\times \] n, where W is a small whole number.
\[=\frac{Weight\,\,of\,\,asubs\tan ce\,\,in\,\,grams}{Grammolecular\,\,weight}=\frac{Number\,\,of\,\,molecules}{Avogadro's\,\,number}\]
- Number of moles of a gaseous substance
\[=\frac{Volume\,\,of\,\,the\,\,gas\,\,at\,\,STD\,\,}{Grammolar\,\,volume\,\,}\]
Number of molecules = Number of moles \[\times \] Avogadro’s number
- Weight of a molecules \[\frac{Weight\,\,of\,\,the\,\,substace}{Grannikecykar\,\,weight}\times Avogadro'snumber\]
Atomic weight of gaseous element =\[\frac{Molecular\,\,weight}{Atomicity}\]
- Weight of the substance = Number of mole \[\times \] gram molecular weight
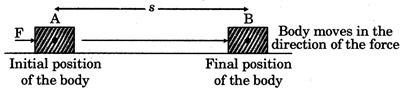 Fig.3.1.
Work done = Force x Displacement (in the direction of force)
\[W=F\times s\]
The work done on the block (or any other object) by a constant force is equal to the product of the magnitude of the more...
Fig.3.1.
Work done = Force x Displacement (in the direction of force)
\[W=F\times s\]
The work done on the block (or any other object) by a constant force is equal to the product of the magnitude of the more... 