Category : NEET
Second Law of Thermodynamics
All the limitations of the first law of thermodynamics can be remove by the second law of thermodynamics. This law is generalisation of certain experiences about heat engines and refrigerators. It has been stated in a number of ways, but all the statements are logically equivalent to one another.
(1) Statements of the law
(i) Kelvin statement : ?It is impossible to derive a continuous supply of work by cooling a body to a temperature lower than that of the coldest of its surroundings.?
(ii) Clausius statement : ?It is impossible for a self acting machine, unaided by any external agency, to convert heat from one body to another at a higher temperature or Heat cannot itself pass from a colder body to a hotter body, but tends invariably towards a lower thermal level.?
(iii) Ostwald statement : ?It is impossible to construct a machine functioning in cycle which can convert heat completely into equivalent amount of work without producing changes elsewhere, i.e., perpetual motions are not allowed.?
(iv) Carnot statement: ?It is impossible to take heat from a hot reservoir and convert it completely into work by a cyclic process without transferring a part of it to a cold reservoir.?
(2) Proof of the law : No rigorous proof is available for the second law. The formulation of the second law is based upon the observations and has yet to be disproved. No deviations of this law have so far been reported. However, the law is applicable to cyclic processes only.
Third Law of thermodynamics
This law was first formulated by German chemist Walther Nernst in 1906. According to this law,
?The entropy of all perfectly crystalline solids is zero at the absolute zero temperature. Since entropy is a measure of disorder, it can be interpreted that at absolute zero, a perfectly crystalline solid has a perfect order of its constituent particles.?
The most important application of the third law of thermodynamics is that it helps in the calculation of absolute entropies of the substance at any temperature T.
\[S=2.303{{C}_{p}}\log T\]
Where \[Cp\]the heat capacity of the substance at constant is pressure and is supposed to remain constant in the range of 0 to T.
Limitations of the law
(1) Glassy solids even at 0K has entropy greater than zero.
(2) Solids having mixtures of isotopes do not have zero entropy at 0K. For example, entropy of solid chlorine is not zero at 0K.
(3) Crystals of Co, N2O, NO, H2O, etc. do not have perfect order even at 0K thus their entropy is not equal to zero.
Entropy and Entropy change
(1) Definition : Entropy is a thermodynamic state quantity which is a measure of randomness or disorder of the molecules of the system.
Entropy is represented by the symbol ?S?. It is difficult to define the actual entropy of a system. It is more convenient to define the change of entropy during a change of state.
The entropy change of a system may be defined as the integral of all the terms involving heat exchanged (q) divided by the absolute temperature (T) during each infinitesimally small change of the process carried out reversibly at constant temperature.
\[\Delta S={{S}_{final}}-{{S}_{initial}}=\frac{{{q}_{rev}}}{T}\]
If heat is absorbed, then \[\Delta S=+ve\]and if heat is evolved, then \[\Delta S=-ve.\]
(2) Units of entropy : Since entropy change is expressed by a heat term divided by temperature, it is expressed in terms of calorie per degree, i.e.,cal deg-1 . In SI units, the entropy is expressed in terms of joule per degree Kelvin, i.e., \[J{{K}^{-1}}\].
(3) Characteristics of entropy : The important characteristics of entropy are summed up below
(i) Entropy is an extensive property. Its value depends upon the amount of the substance present in the system.
(ii) Entropy of a system is a state function. It depends upon the state variables \[(T,p,V,n)\].
(iii) The change in entropy in going from one state to another is independent of the path.
(iv) The change in entropy for a cyclic process is always zero.
(v) The total entropy change of an isolated system is equal to the entropy change of system and entropy change of the surroundings. The sum is called entropy change of universe.
\[\Delta {{S}_{\text{universe}}}=-\Delta {{S}_{sys}}+\Delta {{S}_{Surr}}\]
(a) In a reversible process, \[\Delta {{S}_{universe}}=0\]and, therefore
\[\Delta {{S}_{sys}}=-\Delta {{S}_{Surr}}\]
(b) In an irreversible process, \[\Delta {{S}_{universe}}>0\]. This means that there is increase in entropy of universe is spontaneous changes.
(vi) Entropy is a measure of unavailable energy for useful work.
Unavailable energy = Entropy × Temperature
(vii) Entropy, S is related to thermodynamic probability (W) by the relation,
\[S=k\,{{\log }_{e}}W\,\,\text{and}\,\,\text{S}=\text{ 2}\text{.303k lo}{{\text{g}}_{\text{10}}}W\] ; where, k is Boltzmann's constant
(4) Entropy changes in system & surroundings and total entropy change for Exothermic and Endothermic reactions : Heat increases the thermal motion of the atoms or molecules and increases their disorder and hence their entropy. In case of an exothermic process, the heat escapes into the surroundings and therefore, entropy of the surroundings increases on the other hand in case of endothermic process, the heat enters the system from the surroundings and therefore. The entropy of the surroundings decreases.
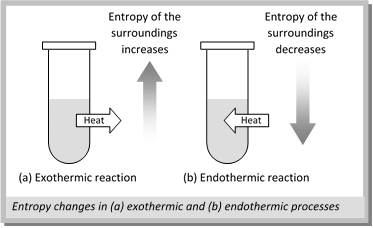
In general, there will be an overall increase of the total entropy (or disorder) whenever the disorder of the surroundings is greater than the decrease in disorder of the system. The process will be spontaneous only when the total entropy increases.
(5) Entropy change during phase transition : The change of matter from one state (solid, liquid or gas) to another is called phase transition. Such changes occur at definite temperature such as melting point (solid to liquid). boiling point (liquid to vapours) etc, and are accompanied by absorption or evolution of heat.
When a solid changes into a liquid at its fusion temperature, there is absorption of heat (latent heat). Let \[\Delta {{H}_{f}}\]be the molar heat of fusion. The entropy change will be
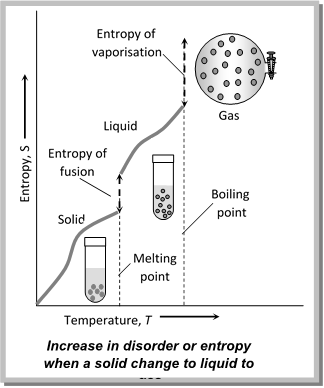
\[\Delta {{S}_{f}}=\frac{\Delta {{H}_{f}}}{{{T}_{f}}}\]
Similarly, if the latent heat of vaporisation and sublimation are denoted by \[\Delta {{H}_{vap}}\]and \[\Delta {{H}_{sub}},\]respectively, the entropy of vaporisation and sublimation are given by
\[\Delta {{S}_{vap}}=\frac{\Delta {{H}_{vap}}}{{{T}_{b}}}\] and \[\Delta {{S}_{sub}}=\frac{\Delta {{H}_{sub}}}{{{T}_{s}}}\]
Since \[\Delta {{H}_{f}},\Delta {{H}_{vap}}\]and \[\Delta {{H}_{Sub}}\] are all positive, these processes are accompanied by increase of entropy.
The reverse processes are accompanied by decrease in entropy.
Note: Entropy increases not only in phase transition but also when the number of moles of products is greater than the number of moles of reactants. (\[{{n}_{\text{product}}}>{{n}_{\text{reactant}}}\,i.e.\Delta n=+ve\])
(6) Entropy change for an ideal gas : In going from initial to final state, the entropy change, \[\Delta S\]for an ideal gas is given by the following relations,
(i) When T and V are two variables, \[\Delta S=n{{C}_{v}}\,\,\,\ln \,\,\frac{{{T}_{2}}}{{{T}_{1}}}+nR\,\,\ln \,\,\frac{{{V}_{2}}}{{{V}_{1}}}\]. Assuming \[{{C}_{v}}\]is constant
(ii) When T and p are two variables, \[\Delta S=n{{C}_{P}}\,\,\ln \,\frac{{{T}_{2}}}{{{T}_{1}}}-nR\,\,\ln \,\,\frac{{{p}_{2}}}{{{p}_{1}}}\]. Assuming \[{{C}_{p}},\]is constant
(a) Thus, for an isothermal process (T constant), \[\Delta S=nR\,\,\ln \frac{{{V}_{2}}}{{{V}_{1}}}\,or=-nR\,\,\,\ln \frac{{{p}_{2}}}{{{p}_{1}}}\]
(b) For isobaric process (p constant), \[\Delta S=n\,{{C}_{p}}\,\ln \frac{{{T}_{2}}}{{{T}_{1}}}\]
(c) For isochoric process (V constant), \[\Delta S=n\,{{C}_{v}}\,\ln \frac{{{T}_{2}}}{{{T}_{1}}}\]
(d) Entropy change during adiabatic expansion: In such process q=0 at all stages. Hence \[\Delta S=0\]. Thus, reversible adiabatic processes are called isoentropic process.
You need to login to perform this action.
You will be redirected in
3 sec
