 Structure and Function
Structure and Function
 This results in existence of a wide variety of plant and animal species in their natural environments, which is the conservationists. Who are mainly concerned about indiscriminate destruction of rainforests and other habitats?
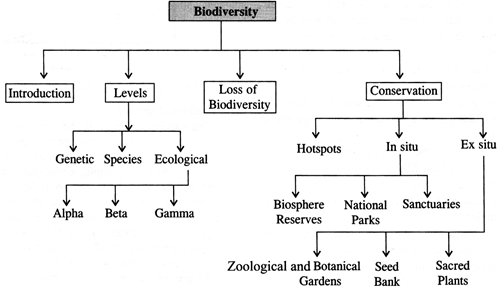
Important Levels of Biodiversity
This results in existence of a wide variety of plant and animal species in their natural environments, which is the conservationists. Who are mainly concerned about indiscriminate destruction of rainforests and other habitats?
Important Levels of Biodiversity
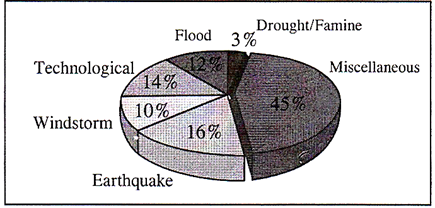 A World Scenario: Reported Deaths from all Disasters (1992-2001)
Indian scenario
The scenario in India is no different from the global context. The super cyclone of Orissa (1999), the Gujarat earthquake (2001) and the recent Tsunami (2004) affected millions across the country leaving behind a trail of heavy loss of life, property and livelihood. Table given below shows a list of some of the major disasters that have caused colossal impact on the community.
A World Scenario: Reported Deaths from all Disasters (1992-2001)
Indian scenario
The scenario in India is no different from the global context. The super cyclone of Orissa (1999), the Gujarat earthquake (2001) and the recent Tsunami (2004) affected millions across the country leaving behind a trail of heavy loss of life, property and livelihood. Table given below shows a list of some of the major disasters that have caused colossal impact on the community.
| S. No. | Disaster | Impact |
| Cyclone | ||
| 1. | more...
Climate Change
Introduction
The year 2015-16 was important for climate change both at domestic and global level. It was started with the groundwork of the third National Communication (NATCOM) under the United Nations Framework Convention on Climate Change (UNFCCC) and the release of the Biennial Update Reports (BURs). It has been clear that human influence is there in the climate system and the recent anthropogenic emissions of greenhouse gases are the highest in history. Recent climate changes have had widespread impacts on human and natural systems.
Change in the Climate System
Warming of the climate system is clear and since the 1950s, many of the observed changes are:
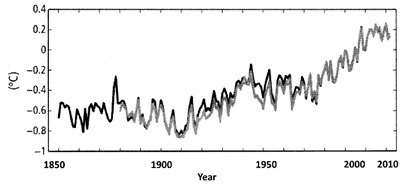 Note: Annually and globally averaged combined land and ocean surface temperature anomalies relative to the average over the period 1986 to 2005. Shades indicate different data sets.
Source: IPCCC +
Figure 1 (b): Globally averaged sea level change
Note: Annually and globally averaged combined land and ocean surface temperature anomalies relative to the average over the period 1986 to 2005. Shades indicate different data sets.
Source: IPCCC +
Figure 1 (b): Globally averaged sea level change
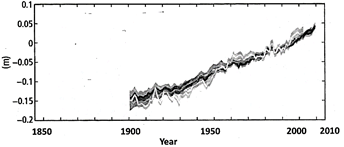 Note: Annually and globally averaged sea level change relative to the average over the period 1986 to 2005 in the
Longest - running dataset. Shades indicate different data sets. All datasets are aligned to have the same value in 1993, the first year of satellite altimetry data. Where assessed, uncertainties are indicated by shades.
Source: IPCC
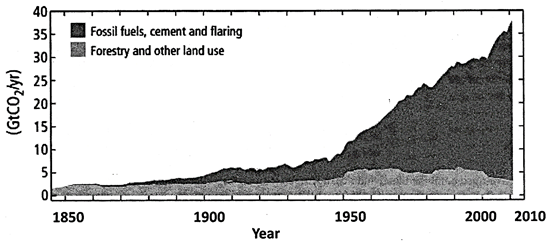
Figure 1 (c): Global anthropogenic \[C{{O}_{2}}\] emissions
Quantitative information of \[C{{H}_{4}}\]and \[{{N}_{2}}O\]emission time series from 1850 to 1970 is limited
Note: Annually and globally averaged sea level change relative to the average over the period 1986 to 2005 in the
Longest - running dataset. Shades indicate different data sets. All datasets are aligned to have the same value in 1993, the first year of satellite altimetry data. Where assessed, uncertainties are indicated by shades.
Source: IPCC
Figure 1 (c): Global anthropogenic \[C{{O}_{2}}\] emissions
Quantitative information of \[C{{H}_{4}}\]and \[{{N}_{2}}O\]emission time series from 1850 to 1970 is limited
 Causes of climate change
Causes of climate change
Environment Management
Introduction
Environmental management system (EMS) refers to the management of an organization's environmental, programs in a comprehensive, systematic, planned and documented manner. It includes the organizational structure, planning and resources for developing, implementing and maintaining policy for environmental protection.
More formally, EMS is "a system and database which integrates procedures and processes for training of personnel, monitoring, summarizing, and reporting of specialized environmental performance information to internal and external stakeholders of a firm.
Environment Management System
The most widely used standard on which an EMS is based
International Organization for Standardization (ISO) 14001. Alternatives include the EMAS.
An environmental management information system (EMIS) is an information technology solution for tracking environmental data for a company as part of their overall environmental management system.
An EMS can also be classified as
Sustainable Development
Introduction
Sustainable development aims at meeting the basic needs of all people in general and the poor majority in particular- their employment, food, energy, water, housing, etc., by ensuring the growth of agriculture, manufactures, power and services with due consideration for environmental concerns.
Over the past two decades, economic growth has lifted more than 660 million people out of poverty and has raised the income levels of millions more, but too often it has come at the expense of the environment and poor communities.
Through a variety of market, policy, and institutional failures. Earth's natural capital has been used in ways that are economically inefficient and wasteful, without sufficient reckoning of the true costs of resource depletion. The burning of fossil fuels supported rapid growth for decades but set up dangerous consequences, with climate change today threatening to roll back decades of development progress. At the same time, growth patterns have left hundreds of millions of people behind: 1.2 billion still lack access to electricity, 870 million are malnourished, and 780 million are still without access to clean, safe drinking water.
Sustainable development recognizes that growth must be both inclusive and environmentally sound to reduce poverty and build shared prosperity for today's population and to continue 10 meet the needs of future generations. It is efficient with resources and carefully planned to deliver both immediate and long-term benefits for people, planet, and prosperity.
The three pillars of sustainable development - economic growth, environmental stewardship, and social inclusion - carry across all sectors of development, from cities facing rapid urbanization to agriculture, infrastructure, energy development and use, water availability, and transportation. Cities are embracing low-carbon growth and public transportation. Farmers are picking up the practices of climate-smart agriculture. Countries are recognizing the value of their natural resources, and industries are realizing how much they can save through energy and supply chain efficiency.
Concept of Sustainable Development
The term was used by the Brundtland Commission which coined what has become the most often-quoted definition of sustainable development as development that "meets the needs of the present without compromising the ability of future generations to meet their own needs. Sustainable development implies economic growth together with the protection of environmental quality, each reinforcing the other. It is maintaining a delicate balance between the human need to improve lifestyles and preserving natural and cultural ecosystems. The field of sustainable development can be conceptually broken into three constituent parts: environmental sustainability, economic sustainability and socio-political sustainability. The essence of this form of development is a stable relationship between human activities and the natural world, which does not diminish the prospects for future generations to enjoy a quality of life at least as good as our own.
Participatory democracy is a prerequisite for achieving sustainable development.
 The linkage between environment and development was globally recognized in 1980, when more...
The linkage between environment and development was globally recognized in 1980, when more...
Terms, Conventions, Policies & Reports
Important Terms
Adaptive radiation:
Evolutionary diversification of a generalized ancestral form with production of a number of adaptively specialized forms. Closely related species look very different, as a result of having adapted to widely different ecological niches.
Agro forestry: An ecologically based farming system that, through the integration of trees in farms, increases social, environmental and economic benefits to land users.
Alien's Rule: Warm-blooded animals (endotherms) from colder climates usually have shorter limbs than do endotherms from warmer climates.
Anthropogenic climate change: Climate change with, the presumption of human influence, usually warming.
Alpha diversity: In ecology, alpha diversity (a-diversity) is the mean species diversity in sites or habitats at a local scale. The term was introduced by R. H. Whittaker together with the terms beta diversity (p-diversity) and gamma diversity (y-diversity).
Allee effect: Concept in population ecology that describes the positive relation between the size of a given population and its growth.
Bagasse: The fibrous residue of sugar cane milling used as a fuel to produce steam in sugar mills.
Beta diversity: (p-diversity or true beta diversity) is the ratio between regional and local species diversity.
Blue water: Collectible water from rainfall; the water that falls on roofs and hard surfaces usually flowing into rivers and the sea and recharging the ground water. In nature the global average proportion of total rainfall that is blue water is about 40%
Biodiversity Hotspot: A biodiversity hotspot is an area with unusual concentration of species, many of which are endemic. It is marked by serious threat to its biodiversity by humans. The concept was given in 1988 by Norman Myers.
Carbon diet: A carbon diet refers to reducing the impact on climate change by reducing greenhouse gas principally \[C{{O}_{2}}\] production.
Carpooling: Giving people lifts to help reduce emissions and traffic.
Carbon budget: A measure of carbon inputs and outputs for a particular activity.
Carbon credit: A market-driven way of reducing the impact of greenhouse gas emissions; it allows an agent to benefit financially from an emission reduction. There are two forms of carbon credit, those that are part of national and international trade and those that are purchased by individuals. Internationally, to achieve Kyoto Protocol objectives, 'caps' (limits) on participating country's emissions are established. To meet these limits countries, in turn, set 'caps' (allowances or credits: 1 convertible and transferable credit = 1 metric tonne of \[C{{O}_{2}}\]emissions) for operators. Operators that meet the agreed 'caps' can then sell unused credits to operators who exceed 'caps'. Operators can then choose the most cost-effective way of reducing emissions. Individual carbon credits would operate in a similar way cf. carbon offset.
Carbon footprint: A measure of the carbon emissions that are emitted over the full life cycle of a product or service and usually expressed as grams of \[C{{O}_{2}}-e\]
Carbon taxes: A surcharge on fossil fuels that aims to reduce carbon dioxide emissions.
Clean Development Mechanism (CDM): The Clean Development Mechanism more...
Current Ecological Developments
Introduction
There have been several ecological developments in India and around the world. The ecological changes have profound impact on the flora and fauna of terrestrial and aquatic environments. Ecological balance is necessary for achieving sustainable environment. In this chapter, we will discuss the current ecological developments in India and around the world.
Ecological balance is a theory which highlights that natural conditions, including numbers of various animal and plant species, remain stable on their own through variations over time. The theory, also known as balance of nature, also holds that natural equilibrium can be changed significantly by new species entering an ecosystem, the disappearance of some species, man-made changes to the environment or natural disasters.
Ecological Imbalance in India is governed by the following factors:
(a) Conservation of Land and Soil
(b) Forest density
(c) Utilization of water resources
(d) Mining Practices
(e) Level of Industrial and Atmospheric Pollution,
 Fig: An overview of the goals of ecological balance.
Costal Ecology
Blue Economy
Blue Economy refers to the integration of ocean economy development with the idea of social inclusion, environmental sustainability and innovative, dynamic business models. It is an approach wherein renewable and organic inputs are fed into sustainably designed systems to promote "blue growth". Such "blue growth" has solved the problems of resource scarcity and waste disposal, while ensuring sustainable development that enhances human welfare in an holistic manner. Blue Economy has also led to creating a healthy ocean environment, supporting higher productivity.
The concept of Blue Economy is introduced by entrepreneur Gunter Pauli. Bilateral and multilateral work, involving the environment, energy, defense and food production can be achieved with Blue Economy. The newly set up Blue Economy Strategic Thought Forum India, under the guidance of the National Maritime Foundation, has focuses on multiple ways in which the blue economy will influence human activities. The central principle of the blue economy is the idea of integrating nutrients and energy the way ecosystems do. Cascading energy and nutrients leads to sustainability by reducing or eliminating inputs, such as energy, and eliminating waste.
Coastal Area Conservation
The coastal environment is facing a number of pressures, arising out of the needs of people, and the multiple uses that coastal and marine areas can be put to. Coastal area in India has seen major developmental changes in recent years as given below:
Fig: An overview of the goals of ecological balance.
Costal Ecology
Blue Economy
Blue Economy refers to the integration of ocean economy development with the idea of social inclusion, environmental sustainability and innovative, dynamic business models. It is an approach wherein renewable and organic inputs are fed into sustainably designed systems to promote "blue growth". Such "blue growth" has solved the problems of resource scarcity and waste disposal, while ensuring sustainable development that enhances human welfare in an holistic manner. Blue Economy has also led to creating a healthy ocean environment, supporting higher productivity.
The concept of Blue Economy is introduced by entrepreneur Gunter Pauli. Bilateral and multilateral work, involving the environment, energy, defense and food production can be achieved with Blue Economy. The newly set up Blue Economy Strategic Thought Forum India, under the guidance of the National Maritime Foundation, has focuses on multiple ways in which the blue economy will influence human activities. The central principle of the blue economy is the idea of integrating nutrients and energy the way ecosystems do. Cascading energy and nutrients leads to sustainability by reducing or eliminating inputs, such as energy, and eliminating waste.
Coastal Area Conservation
The coastal environment is facing a number of pressures, arising out of the needs of people, and the multiple uses that coastal and marine areas can be put to. Coastal area in India has seen major developmental changes in recent years as given below:
Constitutional Framework and Citizenship
Introduction
The term constitution is derived from latin word “constituere” which means to “to establish”.
Constitution means a document having a special legal sanctity, which sets out the framework, principles and functions of the government. The idea of constitutionalism suggests ways and means to work out a government form, which exercises power and ensures, at the same time, individuals freedom and liberty.
 HISTORICAL BACKGROUND
HISTORICAL BACKGROUND
|