Answer:
(c)
When HCI is passed through water then HCI being a polar covalent compound ,ionises
in water as
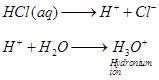
You need to login to perform this action.
You will be redirected in
3 sec
