Answer:
X
is calcium carbonate and the gas evolved is carbon dioxide, when calcium carbon
reacts with acid.
![]() Solution Y is lime water
Solution Y is lime water![]() because,
when
because,
when![]() is passed
through it, it gives the carbonate back as shown by the given equation.
is passed
through it, it gives the carbonate back as shown by the given equation.
![]() The gas evolved at anode during
electrolysis of brine is chlorine (G).
The gas evolved at anode during
electrolysis of brine is chlorine (G).
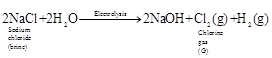 When chlorine gas is passed through dry
When chlorine gas is passed through dry ![]() (Y), it
produces bleaching powder (Z), used for disinfecting drinking water.
(Y), it
produces bleaching powder (Z), used for disinfecting drinking water.
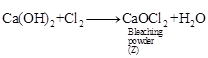 Hence, Z is calcium oxy-chloride (CaOCl2)
or bleaching powder.
Hence, Z is calcium oxy-chloride (CaOCl2)
or bleaching powder.
You need to login to perform this action.
You will be redirected in
3 sec
