Answer:
The
sulphate salt which is used for making different shapes is plaster of Paris.
Its chemical name is calcium sulphate hemihydrate ![]() . The two
formula units of CaSO4 share one molecule of water. As a result, it is soft.
When it is left open for some time, it absorbs moisture from the atmosphere and
forms gypsum which is a hard solid mass.
So, gypsum sets as a hard solid mass and
cannot be used for moulding purposes.
. The two
formula units of CaSO4 share one molecule of water. As a result, it is soft.
When it is left open for some time, it absorbs moisture from the atmosphere and
forms gypsum which is a hard solid mass.
So, gypsum sets as a hard solid mass and
cannot be used for moulding purposes.
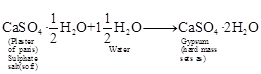 .
.
You need to login to perform this action.
You will be redirected in
3 sec
