Answer:
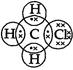
The molecule of chloromethane ![]() consists of three elements i.e., carbon (Z = 6) hydrogen
(Z = 1) and chlorine (Z = 17). Carbon atom has four valence electrons (2, 4); hydrogen has one (1)
while chlorine has seven electrons in the valence shell (2, 8, 7). In order to complete its octet, carbon
shares three valence electrons with three hydrogen atoms while one is shared with the electron of
chlorine atom. The structure of covalent molecule may be written as follows:
consists of three elements i.e., carbon (Z = 6) hydrogen
(Z = 1) and chlorine (Z = 17). Carbon atom has four valence electrons (2, 4); hydrogen has one (1)
while chlorine has seven electrons in the valence shell (2, 8, 7). In order to complete its octet, carbon
shares three valence electrons with three hydrogen atoms while one is shared with the electron of
chlorine atom. The structure of covalent molecule may be written as follows:
 or
or 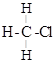
You need to login to perform this action.
You will be redirected in
3 sec
