Answer:
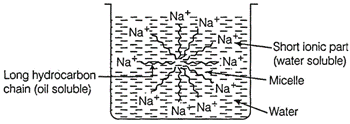
(a) A 'spherical
aggregate of soap molecules' in the soap solution in water is called a 'micelle'.
In a soap micelle, the soap molecules are arranged readily with hydrocarbon ends
directed towards the centre and ionic ends directed outwards.
You need to login to perform this action.
You will be redirected in
3 sec
