Answer:
(a) This is because silver is below hydrogen in the
reactivity series so, cannot displace hydrogen when reacted with acid.
(b) The reaction of Al with dilute HCI
is exothermic i.e., heat is produced in the reaction, hence the temperature of
the reaction mixture rises.
![]() (c) Sodium is a very reactive metal. It
reacts explosively (extremely rapidly) with hydrochloric acid to form sodium
chloride and hydrogen with the evolution of heat too.
H2 gas produced catches fire immediately
(d) Lead is present just above the
hydrogen in the activity series of metals. Hence, it is slightly more reactive
and displace hydrogen from acid very slowly and to a small extent. Therefore,
only bubbles ofH2 are seen to evolve.
(c) Sodium is a very reactive metal. It
reacts explosively (extremely rapidly) with hydrochloric acid to form sodium
chloride and hydrogen with the evolution of heat too.
H2 gas produced catches fire immediately
(d) Lead is present just above the
hydrogen in the activity series of metals. Hence, it is slightly more reactive
and displace hydrogen from acid very slowly and to a small extent. Therefore,
only bubbles ofH2 are seen to evolve.
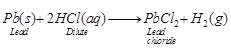
You need to login to perform this action.
You will be redirected in
3 sec
