Answer:
Dark coloured bottles interrupt the path of light
such that light cannot reach silver chloride in the bottles and its
decomposition is prevented. It is known that silver chloride decomposes to
silver and chlorine in the presence of light. This is shown in the reaction
given below
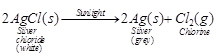 Hence, it is stored in dark coloured
bottles.
Hence, it is stored in dark coloured
bottles.
You need to login to perform this action.
You will be redirected in
3 sec
