Answer:
The position of zinc in the reactivity series is
above hydrogen whereas that of copper is below hydrogen. It means that Zn oxidizes
(loses electrons) more easily than hydrogen whereas copper does not do so. So,
copper does not displace Hg from dilute acids. The reaction is
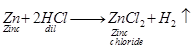
![]()
You need to login to perform this action.
You will be redirected in
3 sec
