Answer:
(a) Silver article turns black when kept in the air
because silver article reacts with sulphur compounds such as hydrogen sulphide
(H2S) present in air. The phenomenon is called corrosion. For silver
particularly, it is called tarnishing of silver.
(b) The black substance formed is silver
sulphide (![]() ).
).
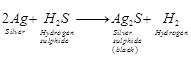
You need to login to perform this action.
You will be redirected in
3 sec
