Answer:
(a) 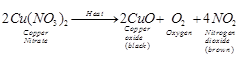 (b) X is nitrogen dioxide gas (NO2).
(c) This is a thermal decomposition
reaction.
(d) The aqueous solution of the gas is
acidic because it is an oxide of a non-metal, so its pH range would be in
between 1 to 7.
(b) X is nitrogen dioxide gas (NO2).
(c) This is a thermal decomposition
reaction.
(d) The aqueous solution of the gas is
acidic because it is an oxide of a non-metal, so its pH range would be in
between 1 to 7.
You need to login to perform this action.
You will be redirected in
3 sec
