Answer:
(a) Test for CO2 gas (lime water test)
When CO2 gas is passed through lime water, it turns milky due to the
formation of insoluble calcium carbonate.
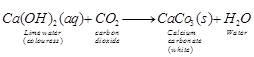 If the gas is in excess, milkiness
disappears due to the formation of soluble calciumbi carbonate.
If the gas is in excess, milkiness
disappears due to the formation of soluble calciumbi carbonate.
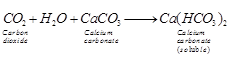 (b) Test for SO2gas
(i) Sulphur dioxide gas turns moist
litmus paper from blue to red because SO, is acidic in
(ii) Sulphur dioxide gas turns acidified
potassium dichromate (vi) solution from orange to green.
(b) Test for SO2gas
(i) Sulphur dioxide gas turns moist
litmus paper from blue to red because SO, is acidic in
(ii) Sulphur dioxide gas turns acidified
potassium dichromate (vi) solution from orange to green.
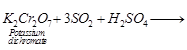
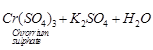 (c) Test for O2 gas When a
wooden splinter is brought near the mouth of the gas jar containing oxygen gas,
it burns brightly as oxygen is the supporter of combustion.
(c) Test for O2 gas When a
wooden splinter is brought near the mouth of the gas jar containing oxygen gas,
it burns brightly as oxygen is the supporter of combustion.
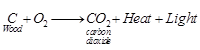 (d) Test for K, gas Hydrogen gas burns
in the presence of air or oxygen with a pop sound when a burning candle is
brought near it.
(d) Test for K, gas Hydrogen gas burns
in the presence of air or oxygen with a pop sound when a burning candle is
brought near it.
You need to login to perform this action.
You will be redirected in
3 sec
