Answer:
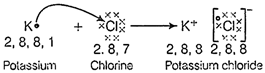
Atomic number
of A = 19
Electronic
configuration is 2, 8, 8,1
Hence,
element A is metal potassium (K) and,
Atomic
number of B = 17
Electronic configuration
is 2, 8,7
It is a
non-metal, chlorine (Cl).
So, the
electron dot structure of KCI is
 The bond
formed between K+ and Cl- is ionic bond and formula of
the product formed
K+ Cl?or
KCI.
The bond
formed between K+ and Cl- is ionic bond and formula of
the product formed
K+ Cl?or
KCI.
You need to login to perform this action.
You will be redirected in
3 sec
