Answer:
(a) The electronic
configuration of element 'X' with atomic number 17 is 2, 8, 7. Since, it has7
valence electrons. Therefore, it lies in group 17 (10 + 7). Further, since in element
X, third shell is being filled, it lies in third period. In other words, X is
chlorine.
The electronic
configuration of element T with atomic number 20 is 2, 8, 8, 2. Since, it has 2
valence electrons, it lies in group 2. Further, since in element Y, fourth
shell is being filled, it lies in 4thperiod. In other words, Y is
calcium.
(b) Since,
element X (i.e., Cl) has seven electrons in the valence shell and needs one
more electron to complete its octet. Therefore it is a non-metal. Further,
since element Y has two electrons in the valence shell which it can easily lose
to achieve the stable electronic configuration of the nearest inert gas,
therefore, it is a metal.
(c) Since,
element Y (i.e., Ca) is a metal, therefore, its oxide (i.e., CaO) must be basic
in nature. Further, since metals and non-metals form ionic compounds,
therefore, the nature of bonding in calcium oxide is ionic.
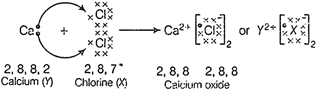
(d) Electronic
configuration of![]() [valence
electrons = 2] electronic configuration of
[valence
electrons = 2] electronic configuration of ![]() [valence
electrons =7]. The electron dot structure of divalent metal halide, i.e., CaCl2
is
[valence
electrons =7]. The electron dot structure of divalent metal halide, i.e., CaCl2
is
You need to login to perform this action.
You will be redirected in
3 sec
