Answer:
The second period elements with
their symbols, atomic numbers and ionisation enthalpies are given below :
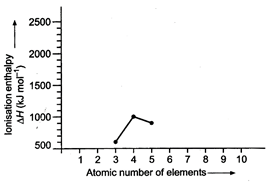
In a period, the value of
ionisation enthalpy increases from left to right with breaks where the atoms
have somewhat stable configurations. Be and N have higher values than expected.
Be has fully filled orbital while N has half filled orbitals.
Be: \[1{{s}^{2}},2{{s}^{2}}\]
N : \[1{{s}^{2}},2{{s}^{2}},2p_{x}^{1},2p_{y}^{1}2p_{z}^{1}\]
Complete the graph yourself and
compare with the graph given in problem 38.
Symbol
Li
Be
B
C
N
O
F
Ne
At. Number
3
4
5
6
7
8
9
10
\[\Delta {{H}_{1}}(kJ\,mo{{l}^{-1}})\]
520
899
801
1086
1402
1314
1681
2080
You need to login to perform this action.
You will be redirected in
3 sec
