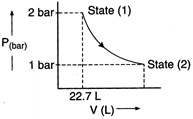
Answer:
In isothermal condition
\[{{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}\]
\[\frac{{{V}_{2}}}{{{V}_{1}}}=\frac{{{P}_{1}}}{{{P}_{2}}}=\frac{2}{1}=2\]
\[w=2.303nRT\,\log
\,\left( \frac{{{P}_{1}}}{{{P}_{2}}} \right)\]
\[=2.303\times
1\times 8.314\times 298{{\log }_{10}}2\]
\[=1717.63J\]
\[{{w}_{\exp
}}=-1717.63J\]
You need to login to perform this action.
You will be redirected in
3 sec
