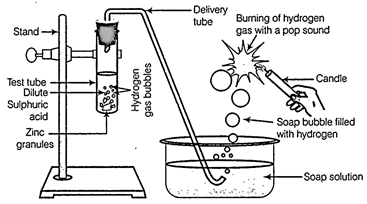
 (a) In
place of zinc granules, same amount of zinc dust is taken in the test tube.
(b)
Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) In
place of zinc, copper turnings are taken.
(d)
Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is
heated.
(a) In
place of zinc granules, same amount of zinc dust is taken in the test tube.
(b)
Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) In
place of zinc, copper turnings are taken.
(d)
Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is
heated.
Answer:
(a)
It same amount of zinc dust is taken in the test tube then the reaction will
be comparatively faster and hydrogen gas will evolve with greater speed. It is
because dust has larger surface area than zinc granules.
(b) With dilute hydrochloric acid,
almost same amount of gas is evolved.
(c) With copper turnings, hydrogen gas
will not evolve because copper is less reactive, it does no react with dil.![]() or dil.
HCI. Hence, no reaction will take place.
(d) Zinc also react with NaOH, So, if
sodium hydroxide is taken, then hydrogen gas will evolved.
or dil.
HCI. Hence, no reaction will take place.
(d) Zinc also react with NaOH, So, if
sodium hydroxide is taken, then hydrogen gas will evolved.
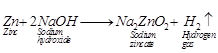
You need to login to perform this action.
You will be redirected in
3 sec
