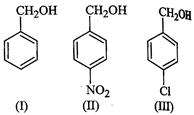
 (a)
(a)Answer:
(c)
All the three benzyl alcohols react with HBr/HCl through intermediate formation
of carbocations. Obviously more stable the carbocation, more reactive is the
alcohol. Now electron-withdrawing groups, i.e., ![]() Cl,
etc. decrease the stability of carbocations. Since the -
Cl,
etc. decrease the stability of carbocations. Since the - ![]() group is a stronger
electron- withdrawing group than - Cl, therefore, stability of carbocations
increases in the order
group is a stronger
electron- withdrawing group than - Cl, therefore, stability of carbocations
increases in the order
![]() Therefore, reactivity of the benzyl alcohols increases in the same order,
i.e., II < III < I. Thus, option (c) is correct.
Therefore, reactivity of the benzyl alcohols increases in the same order,
i.e., II < III < I. Thus, option (c) is correct.
You need to login to perform this action.
You will be redirected in
3 sec
