Answer:
![]() is a linear molecule. The
dipole moments of the two C = O bonds being equal and opposite cancel out each
other and hence
is a linear molecule. The
dipole moments of the two C = O bonds being equal and opposite cancel out each
other and hence ![]() is a non-polar
molecule.
is a non-polar
molecule.
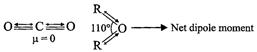 In contrast, in R?O?R molecule, the two dipoles of the R?O bonds are
inclined to each other at angle of 110° i.e, the two dipoles do not cancel out
and hence have a finite resultant. In other words, R?O?R is a polar molcule.
In contrast, in R?O?R molecule, the two dipoles of the R?O bonds are
inclined to each other at angle of 110° i.e, the two dipoles do not cancel out
and hence have a finite resultant. In other words, R?O?R is a polar molcule.
You need to login to perform this action.
You will be redirected in
3 sec
