Answer:
(i) Propanone to propene
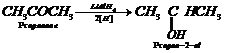
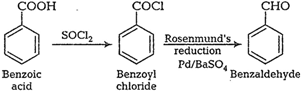
![]() (ii) Benzoic acid to benzaldehyde
(ii) Benzoic acid to benzaldehyde
 (iii) Ethanol to 3-hydroxybutanal
(iii) Ethanol to 3-hydroxybutanal
![]()
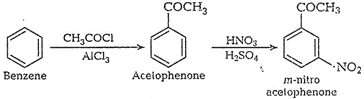
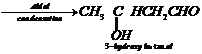 (iv) Benzene to m-nitroacetophenone
(iv) Benzene to m-nitroacetophenone
 (v) Benzaldehyde to benzophenone
(v) Benzaldehyde to benzophenone
![]() (vi) Bromobenzene to 1-phenylethanol
(vi) Bromobenzene to 1-phenylethanol
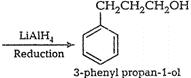
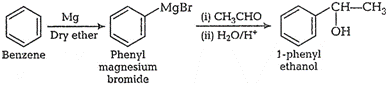 (vii) Benzaldehyde to 3-phenyl propan-1-ol
(vii) Benzaldehyde to 3-phenyl propan-1-ol
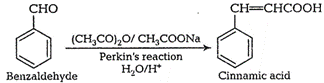
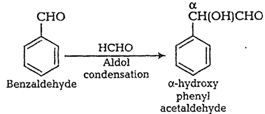
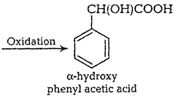
 (viii) Benzaldehyde to
(viii) Benzaldehyde to ![]() -hydroxy phenyl acetic acid
-hydroxy phenyl acetic acid
 (ix) Benzoic acid to m-nitrobenzyl alcohol
(ix) Benzoic acid to m-nitrobenzyl alcohol
You need to login to perform this action.
You will be redirected in
3 sec
