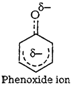
Answer:
1. Phenoxide ion has non-equivalent resonance structures
inwhich the negative charge is at the less electronegative carbonatom.
2. The negative charge is delocalized
over two electronegative oxygen atoms in carboxylate ion whereas in phenoxide
ion the negative charge less effectively delocalized over one oxygen atom and
less electronegative carbon atoms.
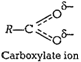
You need to login to perform this action.
You will be redirected in
3 sec
